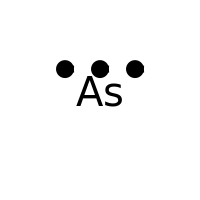
DISCLAIMER
ExHuMId is a knowledgebase compiled through manual curation of published scientific literature wherein environmental contaminants were detected in human milk samples across India. We are not responsible for any errors in published scientific articles from where the human milk exposome specific to India was compiled. We are also not responsible for any omission of published scientific articles containing information on human milk exposome specific to India. Importantly, our primary goal to build this resource was to enable future research on human exposome studies specific to India. Hence, the views expressed in this work are solely based on our scientific understanding of the topic, and they do not necessarily reflect the views or policies of our employers or funders.