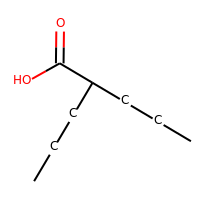
Valproic acid
| Chemical identification | |
|---|---|
| Pubchem identifier | 3121 |
| CAS identifier | 99-66-1 |
| IUPAC name | Valproic acid |
| Structure |  2D:2D MOL2D MOL22D SDF 3D:3D MOL3D MOL23D SDF3D PDB3D PDBQT |
| SMILES | CCCC(CCC)C(=O)O |
| InChI | InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) |
| InChIKey | NIJJYAXOARWZEE-UHFFFAOYSA-N |
| Synonyms | 2-Propylpentanoic acid; 2-Propylvaleric acid; 4-Heptanecarboxylic acid; Abbott 44090; Acetic acid, dipropyl-; Acide valproique; Acido valproico; Acidum valproicum; Depakene; Depakin; Depakine; Di-n-propylacetic acid; Di-n-propylessigsaure; Dipropylacetate; Dipropylacetic acid; Ergenyl; Kyselina 2-propylvalerova; Mylproin; Myproic acid; n-Dipropylacetic acid; Pentanoic acid, 2-propyl-; Propylvaleric acid; Stavzor; Valeric acid, 2-propyl-; Valproate; Valproic acid |
| External identifiers | D014635 |