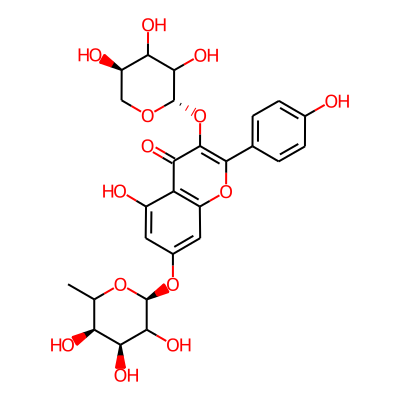
Summary
SMILES: Oc1ccc(cc1)c1oc2cc(O[C@@H]3OC(C)[C@@H]([C@@H](C3O)O)O)cc(c2c(=O)c1O[C@@H]1OC[C@H](C(C1O)O)O)OInChI: InChI=1S/C26H28O14/c1-9-17(30)20(33)22(35)26(37-9)38-12-6-13(28)16-15(7-12)39-23(10-2-4-11(27)5-3-10)24(19(16)32)40-25-21(34)18(31)14(29)8-36-25/h2-7,9,14,17-18,20-22,25-31,33-35H,8H2,1H3/t9?,14-,17+,18?,20+,21?,22?,25+,26+/m1/s1InChIKey: DQBVFTJNUYZVQL-WIOJAONRSA-N
DeepSMILES: Occcccc6))cocccO[C@@H]OCC)[C@@H][C@@H]C6O))O))O))))))ccc6c=O)c%10O[C@@H]OC[C@H]CC6O))O))O)))))))))O
Scaffold Graph/Node/Bond level: O=c1c(OC2CCCCO2)c(-c2ccccc2)oc2cc(OC3CCCCO3)ccc12
Scaffold Graph/Node level: OC1C2CCC(OC3CCCCO3)CC2OC(C2CCCCC2)C1OC1CCCCO1
Scaffold Graph level: CC1C2CCC(CC3CCCCC3)CC2CC(C2CCCCC2)C1CC1CCCCC1
Functional groups: CO; c=O; cO; cO[C@@H](C)OC; coc
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Flavonoids
ClassyFire Subclass: Flavonoid glycosides
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Flavonoids
NP Classifier Class: Flavonols
Synonymous chemical names:lepidoside, lepidoside(3-d-xylosyl-7-α-rhamnosyl kaempferol)
External chemical identifiers:CID:44258931
Chemical structure download