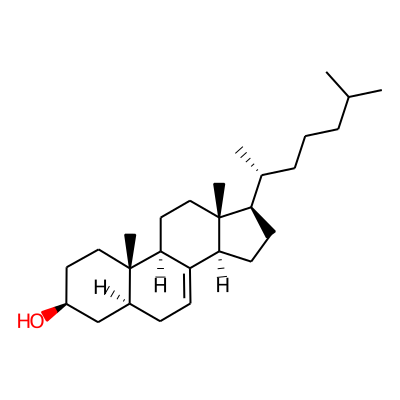
Summary
SMILES: CC(CCC[C@H]([C@H]1CC[C@@H]2[C@]1(C)CC[C@H]1C2=CC[C@@H]2[C@]1(C)CC[C@@H](C2)O)C)CInChI: InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h10,18-21,23-25,28H,6-9,11-17H2,1-5H3/t19-,20+,21+,23-,24+,25+,26+,27-/m1/s1InChIKey: IZVFFXVYBHFIHY-SKCNUYALSA-N
DeepSMILES: CCCCC[C@H][C@H]CC[C@@H][C@]5C)CC[C@H]C6=CC[C@@H][C@]6C)CC[C@@H]C6)O)))))))))))))))))C)))))C
Scaffold Graph/Node/Bond level: C1=C2C3CCCC3CCC2C2CCCCC2C1
Scaffold Graph/Node level: C1CCC2C(C1)CCC1C3CCCC3CCC21
Scaffold Graph level: C1CCC2C(C1)CCC1C3CCCC3CCC21
Functional groups: CC=C(C)C; CO
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Steroids and steroid derivatives
ClassyFire Subclass: Cholestane steroids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Steroids
NP Classifier Class: Cholestane steroids
Synonymous chemical names:cholest-7-en-3-ol, cholest-7-en-3beta-ol, cholesta-7-enol, δ7-cholesten-3β-ol
External chemical identifiers:CID:65728; ChEMBL:CHEMBL3138639; ChEBI:17168; ZINC:ZINC000004095573; SureChEMBL:SCHEMBL187737; MolPort-004-956-484
Chemical structure download