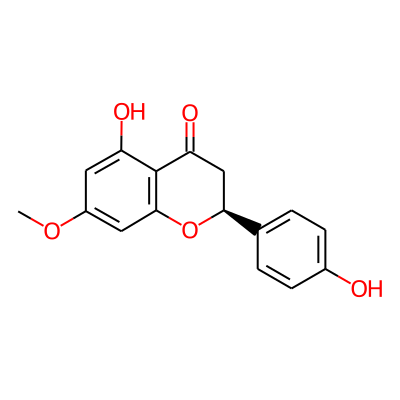
Summary
SMILES: COc1cc2O[C@@H](CC(=O)c2c(c1)O)c1ccc(cc1)OInChI: InChI=1S/C16H14O5/c1-20-11-6-12(18)16-13(19)8-14(21-15(16)7-11)9-2-4-10(17)5-3-9/h2-7,14,17-18H,8H2,1H3/t14-/m0/s1InChIKey: DJOJDHGQRNZXQQ-AWEZNQCLSA-N
DeepSMILES: COcccO[C@@H]CC=O)c6cc%10)O)))))cccccc6))O
Scaffold Graph/Node/Bond level: O=C1CC(c2ccccc2)Oc2ccccc21
Scaffold Graph/Node level: OC1CC(C2CCCCC2)OC2CCCCC12
Scaffold Graph level: CC1CC(C2CCCCC2)CC2CCCCC12
Functional groups: cC(C)=O; cO; cOC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Flavonoids
ClassyFire Subclass: O-methylated flavonoids
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Flavonoids
NP Classifier Class: Flavanones
Synonymous chemical names:naringenin-7-methyl ether, sakuranetin, sakuranetin(5,4'-dihydroxy-7-methoxyflavone)
External chemical identifiers:CID:73571; ChEMBL:CHEMBL448297; ChEBI:28927; ZINC:ZINC000000338284; FDASRS:3O38P61299; SureChEMBL:SCHEMBL555542; MolPort-039-052-519
Chemical structure download