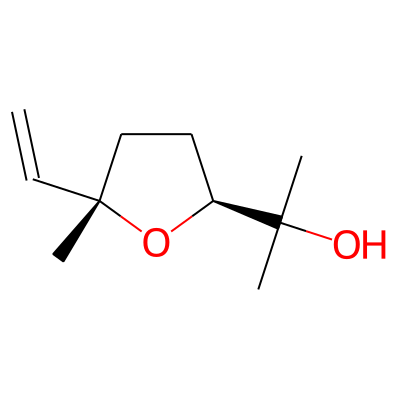
Summary
SMILES: C=C[C@]1(C)CC[C@H](O1)C(O)(C)CInChI: InChI=1S/C10H18O2/c1-5-10(4)7-6-8(12-10)9(2,3)11/h5,8,11H,1,6-7H2,2-4H3/t8-,10+/m0/s1InChIKey: BRHDDEIRQPDPMG-WCBMZHEXSA-N
DeepSMILES: C=C[C@]C)CC[C@H]O5)CO)C)C
Scaffold Graph/Node/Bond level: C1CCOC1
Scaffold Graph/Node level: C1CCOC1
Scaffold Graph level: C1CCCC1
Functional groups: C=CC; CO; COC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Tetrahydrofurans
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Monoterpenoids
NP Classifier Class: Acyclic monoterpenoids
Synonymous chemical names:(e)-linalool oxyde*, frans-linalol oxide, linalol oxide ii (pyranoid), linalool a oxide, linalool oxide (trans), linalool oxide a, linalool oxide i(pyr.), linalool oxide trans, linalool oxide, trans, linalool oxide,trans-, t-linalool oxide, tans-linalool oxide (furanoid), trans-linalol oxide (furanoide), trans-linalol oxide, trans-linalol oxide furanoid, trans-linalol oxide ( furan isomer), trans-linalol oxide ( furanoid), trans-linalol oxide (furanoid), trans-linalol oxide (furanoide), trans-linalool oxide, trans-linalool oxide ( furanoid), trans-linalool oxide (furan form), trans-linalool oxide (furan), trans-linalool oxide (furaneol), trans-linalool oxide (furanoid fom), trans-linalool oxide (furanoid form), trans-linalool oxide (furanoid), trans-linalool oxide (furanoid)*, trans-linalool oxide (furanoidform), trans-linalool oxide (furanoïd), trans-linalool oxide (pyran), trans-linalool oxide acetate (pyr.), trans-linalool oxide*, trans-linalool oxide**, trans-linalool oxide+, trans-linalool oxlde (furanoid), trans-l
External chemical identifiers:CID:6432254; ChEMBL:CHEMBL2252947; ZINC:ZINC000000391154; FDASRS:986J3104O2; SureChEMBL:SCHEMBL1116444
Chemical structure download