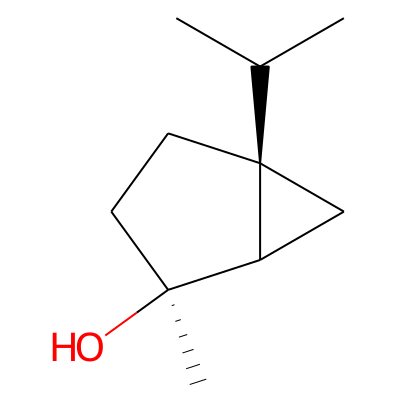
Summary
SMILES: CC([C@@]12CC[C@](C2C1)(C)O)CInChI: InChI=1S/C10H18O/c1-7(2)10-5-4-9(3,11)8(10)6-10/h7-8,11H,4-6H2,1-3H3/t8?,9-,10+/m0/s1InChIKey: KXSDPILWMGFJMM-CBMCFHRWSA-N
DeepSMILES: CC[C@]CC[C@]C5C6))C)O)))))C
Scaffold Graph/Node/Bond level: C1CC2CC2C1
Scaffold Graph/Node level: C1CC2CC2C1
Scaffold Graph level: C1CC2CC2C1
Functional groups: CO
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Monoterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Monoterpenoids
NP Classifier Class: Thujane monoterpenoids
Synonymous chemical names:(e)-sabinenhydrate, irans-sabinene hydrate, linalool + trans-sabinene hydrate, rrans-sabinene hydrate, rruns-sabinenehydrate, rruns-sahinene hydrate, sabinene, trans hydrate, sabinene-trans-hydrate, trans- sabinene hydrate, trans-sa binene hydrate, trans-sabinene hydrate, trans-sabinene hydrat, trans-sabinene hydrate, trans-sabinene hydrate(trans rel.to oh vs. ipp), trans-sabinene hydrate*, trans-sabinene hyrate, trans-sabinenhydrate, trans‐sabinene hydrate
External chemical identifiers:CID:12315151
Chemical structure download