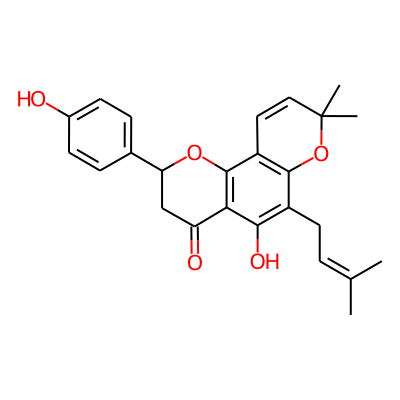
| PaDEL | 2D | nAcid | Number of acidic groups. The list of acidic groups is defined by these SMARTS "$([O;H1]-[C,S,P]=O)" | Acidic Group Count descriptor | 0 |
| PaDEL | 2D | ALogP | Ghose-Crippen LogKow | ALOGP descriptor | 1.43 |
| PaDEL | 2D | ALogp2 | Square of ALogP | ALOGP descriptor | 2.05 |
| PaDEL | 2D | AMR | Molar refractivity | ALOGP descriptor | 70.6 |
| PaDEL | 2D | apol | Sum of the atomic polarizabilities (including implicit hydrogens) | APol descriptor | 65.35 |
| PaDEL | 2D | naAromAtom | Number of aromatic atoms | Aromatic Atoms Count descriptor | 12 |
| PaDEL | 2D | nAromBond | Number of aromatic bonds | Aromatic Bonds Count descriptor | 12 |
| PaDEL | 2D | nAtom | Number of atoms | Atom Count descriptor | 56 |
| PaDEL | 2D | nHeavyAtom | Number of heavy atoms (i.e. not hydrogen) | Atom Count descriptor | 30 |
| PaDEL | 2D | nH | Number of hydrogen atoms | Atom Count descriptor | 26 |
| PaDEL | 2D | nB | Number of boron atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nC | Number of carbon atoms | Atom Count descriptor | 25 |
| PaDEL | 2D | nN | Number of nitrogen atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nO | Number of oxygen atoms | Atom Count descriptor | 5 |
| PaDEL | 2D | nS | Number of sulphur atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nP | Number of phosphorus atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nF | Number of fluorine atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nCl | Number of chlorine atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nBr | Number of bromine atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nI | Number of iodine atoms | Atom Count descriptor | 0 |
| PaDEL | 2D | nX | Number of halogen atoms (F, Cl, Br, I, At, Uus) | Atom Count descriptor | 0 |
| PaDEL | 2D | ATS0m | Broto-Moreau autocorrelation - lag 0 / weighted by mass | Auto correlation descriptor | 4912.86 |
| PaDEL | 2D | ATS1m | Broto-Moreau autocorrelation - lag 1 / weighted by mass | Auto correlation descriptor | 5418.84 |
| PaDEL | 2D | ATS2m | Broto-Moreau autocorrelation - lag 2 / weighted by mass | Auto correlation descriptor | 8422.13 |
| PaDEL | 2D | ATS3m | Broto-Moreau autocorrelation - lag 3 / weighted by mass | Auto correlation descriptor | 9655.66 |
| PaDEL | 2D | ATS4m | Broto-Moreau autocorrelation - lag 4 / weighted by mass | Auto correlation descriptor | 9767.62 |
| PaDEL | 2D | ATS5m | Broto-Moreau autocorrelation - lag 5 / weighted by mass | Auto correlation descriptor | 9282.15 |
| PaDEL | 2D | ATS6m | Broto-Moreau autocorrelation - lag 6 / weighted by mass | Auto correlation descriptor | 9646.61 |
| PaDEL | 2D | ATS7m | Broto-Moreau autocorrelation - lag 7 / weighted by mass | Auto correlation descriptor | 7842.26 |
| PaDEL | 2D | ATS8m | Broto-Moreau autocorrelation - lag 8 / weighted by mass | Auto correlation descriptor | 7147.35 |
| PaDEL | 2D | ATS0v | Broto-Moreau autocorrelation - lag 0 / weighted by van der Waals volumes | Auto correlation descriptor | 12478.05 |
| PaDEL | 2D | ATS1v | Broto-Moreau autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation descriptor | 16048.25 |
| PaDEL | 2D | ATS2v | Broto-Moreau autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation descriptor | 24241.21 |
| PaDEL | 2D | ATS3v | Broto-Moreau autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation descriptor | 28857.65 |
| PaDEL | 2D | ATS4v | Broto-Moreau autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation descriptor | 28745.89 |
| PaDEL | 2D | ATS5v | Broto-Moreau autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation descriptor | 28691.7 |
| PaDEL | 2D | ATS6v | Broto-Moreau autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation descriptor | 28908.48 |
| PaDEL | 2D | ATS7v | Broto-Moreau autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation descriptor | 26996.87 |
| PaDEL | 2D | ATS8v | Broto-Moreau autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation descriptor | 24073.41 |
| PaDEL | 2D | ATS0e | Broto-Moreau autocorrelation - lag 0 / weighted by Sanderson electronegativities | Auto correlation descriptor | 429.95 |
| PaDEL | 2D | ATS1e | Broto-Moreau autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation descriptor | 456.06 |
| PaDEL | 2D | ATS2e | Broto-Moreau autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation descriptor | 788.43 |
| PaDEL | 2D | ATS3e | Broto-Moreau autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1058.89 |
| PaDEL | 2D | ATS4e | Broto-Moreau autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1234.22 |
| PaDEL | 2D | ATS5e | Broto-Moreau autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1134.39 |
| PaDEL | 2D | ATS6e | Broto-Moreau autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1132.45 |
| PaDEL | 2D | ATS7e | Broto-Moreau autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1261.03 |
| PaDEL | 2D | ATS8e | Broto-Moreau autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1081.3 |
| PaDEL | 2D | ATS0p | Broto-Moreau autocorrelation - lag 0 / weighted by polarizabilities | Auto correlation descriptor | 84.5 |
| PaDEL | 2D | ATS1p | Broto-Moreau autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation descriptor | 109.68 |
| PaDEL | 2D | ATS2p | Broto-Moreau autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation descriptor | 166.78 |
| PaDEL | 2D | ATS3p | Broto-Moreau autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation descriptor | 201.05 |
| PaDEL | 2D | ATS4p | Broto-Moreau autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation descriptor | 206.94 |
| PaDEL | 2D | ATS5p | Broto-Moreau autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation descriptor | 205.66 |
| PaDEL | 2D | ATS6p | Broto-Moreau autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation descriptor | 205.14 |
| PaDEL | 2D | ATS7p | Broto-Moreau autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation descriptor | 198.17 |
| PaDEL | 2D | ATS8p | Broto-Moreau autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation descriptor | 183.2 |
| PaDEL | 2D | ATS0i | Broto-Moreau autocorrelation - lag 0 / weighted by first ionization potential | Auto correlation descriptor | 8904.97 |
| PaDEL | 2D | ATS1i | Broto-Moreau autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation descriptor | 8415.37 |
| PaDEL | 2D | ATS2i | Broto-Moreau autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation descriptor | 15330.64 |
| PaDEL | 2D | ATS3i | Broto-Moreau autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation descriptor | 20393.62 |
| PaDEL | 2D | ATS4i | Broto-Moreau autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation descriptor | 25239.94 |
| PaDEL | 2D | ATS5i | Broto-Moreau autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation descriptor | 23119.12 |
| PaDEL | 2D | ATS6i | Broto-Moreau autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation descriptor | 22099.53 |
| PaDEL | 2D | ATS7i | Broto-Moreau autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation descriptor | 25729.47 |
| PaDEL | 2D | ATS8i | Broto-Moreau autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation descriptor | 22661.64 |
| PaDEL | 2D | ATS0s | Broto-Moreau autocorrelation - lag 0 / weighted by I-state | Auto correlation descriptor | 251.12 |
| PaDEL | 2D | ATS1s | Broto-Moreau autocorrelation - lag 1 / weighted by I-state | Auto correlation descriptor | 187.54 |
| PaDEL | 2D | ATS2s | Broto-Moreau autocorrelation - lag 2 / weighted by I-state | Auto correlation descriptor | 308.92 |
| PaDEL | 2D | ATS3s | Broto-Moreau autocorrelation - lag 3 / weighted by I-state | Auto correlation descriptor | 438.78 |
| PaDEL | 2D | ATS4s | Broto-Moreau autocorrelation - lag 4 / weighted by I-state | Auto correlation descriptor | 531.85 |
| PaDEL | 2D | ATS5s | Broto-Moreau autocorrelation - lag 5 / weighted by I-state | Auto correlation descriptor | 425.46 |
| PaDEL | 2D | ATS6s | Broto-Moreau autocorrelation - lag 6 / weighted by I-state | Auto correlation descriptor | 521.31 |
| PaDEL | 2D | ATS7s | Broto-Moreau autocorrelation - lag 7 / weighted by I-state | Auto correlation descriptor | 500.68 |
| PaDEL | 2D | ATS8s | Broto-Moreau autocorrelation - lag 8 / weighted by I-state | Auto correlation descriptor | 424.78 |
| PaDEL | 2D | AATS0m | Average Broto-Moreau autocorrelation - lag 0 / weighted by mass | Auto correlation descriptor | 87.73 |
| PaDEL | 2D | AATS1m | Average Broto-Moreau autocorrelation - lag 1 / weighted by mass | Auto correlation descriptor | 91.84 |
| PaDEL | 2D | AATS2m | Average Broto-Moreau autocorrelation - lag 2 / weighted by mass | Auto correlation descriptor | 81.77 |
| PaDEL | 2D | AATS3m | Average Broto-Moreau autocorrelation - lag 3 / weighted by mass | Auto correlation descriptor | 71.52 |
| PaDEL | 2D | AATS4m | Average Broto-Moreau autocorrelation - lag 4 / weighted by mass | Auto correlation descriptor | 61.05 |
| PaDEL | 2D | AATS5m | Average Broto-Moreau autocorrelation - lag 5 / weighted by mass | Auto correlation descriptor | 62.3 |
| PaDEL | 2D | AATS6m | Average Broto-Moreau autocorrelation - lag 6 / weighted by mass | Auto correlation descriptor | 66.53 |
| PaDEL | 2D | AATS7m | Average Broto-Moreau autocorrelation - lag 7 / weighted by mass | Auto correlation descriptor | 48.71 |
| PaDEL | 2D | AATS8m | Average Broto-Moreau autocorrelation - lag 8 / weighted by mass | Auto correlation descriptor | 49.29 |
| PaDEL | 2D | AATS0v | Average Broto-Moreau autocorrelation - lag 0 / weighted by van der Waals volumes | Auto correlation descriptor | 222.82 |
| PaDEL | 2D | AATS1v | Average Broto-Moreau autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation descriptor | 272 |
| PaDEL | 2D | AATS2v | Average Broto-Moreau autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation descriptor | 235.35 |
| PaDEL | 2D | AATS3v | Average Broto-Moreau autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation descriptor | 213.76 |
| PaDEL | 2D | AATS4v | Average Broto-Moreau autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation descriptor | 179.66 |
| PaDEL | 2D | AATS5v | Average Broto-Moreau autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation descriptor | 192.56 |
| PaDEL | 2D | AATS6v | Average Broto-Moreau autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation descriptor | 199.37 |
| PaDEL | 2D | AATS7v | Average Broto-Moreau autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation descriptor | 167.68 |
| PaDEL | 2D | AATS8v | Average Broto-Moreau autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation descriptor | 166.02 |
| PaDEL | 2D | AATS0e | Average Broto-Moreau autocorrelation - lag 0 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.68 |
| PaDEL | 2D | AATS1e | Average Broto-Moreau autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.73 |
| PaDEL | 2D | AATS2e | Average Broto-Moreau autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.65 |
| PaDEL | 2D | AATS3e | Average Broto-Moreau autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.84 |
| PaDEL | 2D | AATS4e | Average Broto-Moreau autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.71 |
| PaDEL | 2D | AATS5e | Average Broto-Moreau autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.61 |
| PaDEL | 2D | AATS6e | Average Broto-Moreau autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.81 |
| PaDEL | 2D | AATS7e | Average Broto-Moreau autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.83 |
| PaDEL | 2D | AATS8e | Average Broto-Moreau autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation descriptor | 7.46 |
| PaDEL | 2D | AATS0p | Average Broto-Moreau autocorrelation - lag 0 / weighted by polarizabilities | Auto correlation descriptor | 1.51 |
| PaDEL | 2D | AATS1p | Average Broto-Moreau autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation descriptor | 1.86 |
| PaDEL | 2D | AATS2p | Average Broto-Moreau autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation descriptor | 1.62 |
| PaDEL | 2D | AATS3p | Average Broto-Moreau autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation descriptor | 1.49 |
| PaDEL | 2D | AATS4p | Average Broto-Moreau autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation descriptor | 1.29 |
| PaDEL | 2D | AATS5p | Average Broto-Moreau autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation descriptor | 1.38 |
| PaDEL | 2D | AATS6p | Average Broto-Moreau autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation descriptor | 1.41 |
| PaDEL | 2D | AATS7p | Average Broto-Moreau autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation descriptor | 1.23 |
| PaDEL | 2D | AATS8p | Average Broto-Moreau autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation descriptor | 1.26 |
| PaDEL | 2D | AATS0i | Average Broto-Moreau autocorrelation - lag 0 / weighted by first ionization potential | Auto correlation descriptor | 159.02 |
| PaDEL | 2D | AATS1i | Average Broto-Moreau autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation descriptor | 142.63 |
| PaDEL | 2D | AATS2i | Average Broto-Moreau autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation descriptor | 148.84 |
| PaDEL | 2D | AATS3i | Average Broto-Moreau autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation descriptor | 151.06 |
| PaDEL | 2D | AATS4i | Average Broto-Moreau autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation descriptor | 157.75 |
| PaDEL | 2D | AATS5i | Average Broto-Moreau autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation descriptor | 155.16 |
| PaDEL | 2D | AATS6i | Average Broto-Moreau autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation descriptor | 152.41 |
| PaDEL | 2D | AATS7i | Average Broto-Moreau autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation descriptor | 159.81 |
| PaDEL | 2D | AATS8i | Average Broto-Moreau autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation descriptor | 156.29 |
| PaDEL | 2D | AATS0s | Average Broto-Moreau autocorrelation - lag 0 / weighted by I-state | Auto correlation descriptor | 4.48 |
| PaDEL | 2D | AATS1s | Average Broto-Moreau autocorrelation - lag 1 / weighted by I-state | Auto correlation descriptor | 3.18 |
| PaDEL | 2D | AATS2s | Average Broto-Moreau autocorrelation - lag 2 / weighted by I-state | Auto correlation descriptor | 3 |
| PaDEL | 2D | AATS3s | Average Broto-Moreau autocorrelation - lag 3 / weighted by I-state | Auto correlation descriptor | 3.25 |
| PaDEL | 2D | AATS4s | Average Broto-Moreau autocorrelation - lag 4 / weighted by I-state | Auto correlation descriptor | 3.32 |
| PaDEL | 2D | AATS5s | Average Broto-Moreau autocorrelation - lag 5 / weighted by I-state | Auto correlation descriptor | 2.86 |
| PaDEL | 2D | AATS6s | Average Broto-Moreau autocorrelation - lag 6 / weighted by I-state | Auto correlation descriptor | 3.6 |
| PaDEL | 2D | AATS7s | Average Broto-Moreau autocorrelation - lag 7 / weighted by I-state | Auto correlation descriptor | 3.11 |
| PaDEL | 2D | AATS8s | Average Broto-Moreau autocorrelation - lag 8 / weighted by I-state | Auto correlation descriptor | 2.93 |
| PaDEL | 2D | ATSC0c | Centered Broto-Moreau autocorrelation - lag 0 / weighted by charges | Auto correlation descriptor | 0.91 |
| PaDEL | 2D | ATSC1c | Centered Broto-Moreau autocorrelation - lag 1 / weighted by charges | Auto correlation descriptor | -0.37 |
| PaDEL | 2D | ATSC2c | Centered Broto-Moreau autocorrelation - lag 2 / weighted by charges | Auto correlation descriptor | -0.03 |
| PaDEL | 2D | ATSC3c | Centered Broto-Moreau autocorrelation - lag 3 / weighted by charges | Auto correlation descriptor | -0.43 |
| PaDEL | 2D | ATSC4c | Centered Broto-Moreau autocorrelation - lag 4 / weighted by charges | Auto correlation descriptor | 0.59 |
| PaDEL | 2D | ATSC5c | Centered Broto-Moreau autocorrelation - lag 5 / weighted by charges | Auto correlation descriptor | -0.35 |
| PaDEL | 2D | ATSC6c | Centered Broto-Moreau autocorrelation - lag 6 / weighted by charges | Auto correlation descriptor | 0.45 |
| PaDEL | 2D | ATSC7c | Centered Broto-Moreau autocorrelation - lag 7 / weighted by charges | Auto correlation descriptor | -0.49 |
| PaDEL | 2D | ATSC8c | Centered Broto-Moreau autocorrelation - lag 8 / weighted by charges | Auto correlation descriptor | 0.33 |
| PaDEL | 2D | ATSC0m | Centered Broto-Moreau autocorrelation - lag 0 / weighted by mass | Auto correlation descriptor | 1962.43 |
| PaDEL | 2D | ATSC1m | Centered Broto-Moreau autocorrelation - lag 1 / weighted by mass | Auto correlation descriptor | 55.81 |
| PaDEL | 2D | ATSC2m | Centered Broto-Moreau autocorrelation - lag 2 / weighted by mass | Auto correlation descriptor | 777.12 |
| PaDEL | 2D | ATSC3m | Centered Broto-Moreau autocorrelation - lag 3 / weighted by mass | Auto correlation descriptor | -669.03 |
| PaDEL | 2D | ATSC4m | Centered Broto-Moreau autocorrelation - lag 4 / weighted by mass | Auto correlation descriptor | 735.7 |
| PaDEL | 2D | ATSC5m | Centered Broto-Moreau autocorrelation - lag 5 / weighted by mass | Auto correlation descriptor | 462.54 |
| PaDEL | 2D | ATSC6m | Centered Broto-Moreau autocorrelation - lag 6 / weighted by mass | Auto correlation descriptor | -725.82 |
| PaDEL | 2D | ATSC7m | Centered Broto-Moreau autocorrelation - lag 7 / weighted by mass | Auto correlation descriptor | -1549.9 |
| PaDEL | 2D | ATSC8m | Centered Broto-Moreau autocorrelation - lag 8 / weighted by mass | Auto correlation descriptor | -75.44 |
| PaDEL | 2D | ATSC0v | Centered Broto-Moreau autocorrelation - lag 0 / weighted by van der Waals volumes | Auto correlation descriptor | 2883.7 |
| PaDEL | 2D | ATSC1v | Centered Broto-Moreau autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation descriptor | 168.59 |
| PaDEL | 2D | ATSC2v | Centered Broto-Moreau autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation descriptor | 785.33 |
| PaDEL | 2D | ATSC3v | Centered Broto-Moreau autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation descriptor | -1001.95 |
| PaDEL | 2D | ATSC4v | Centered Broto-Moreau autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation descriptor | 656.3 |
| PaDEL | 2D | ATSC5v | Centered Broto-Moreau autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation descriptor | 690.34 |
| PaDEL | 2D | ATSC6v | Centered Broto-Moreau autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation descriptor | -1602.52 |
| PaDEL | 2D | ATSC7v | Centered Broto-Moreau autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation descriptor | -418.68 |
| PaDEL | 2D | ATSC8v | Centered Broto-Moreau autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation descriptor | -912.56 |
| PaDEL | 2D | ATSC0e | Centered Broto-Moreau autocorrelation - lag 0 / weighted by Sanderson electronegativities | Auto correlation descriptor | 4.73 |
| PaDEL | 2D | ATSC1e | Centered Broto-Moreau autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.31 |
| PaDEL | 2D | ATSC2e | Centered Broto-Moreau autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.16 |
| PaDEL | 2D | ATSC3e | Centered Broto-Moreau autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation descriptor | -1.79 |
| PaDEL | 2D | ATSC4e | Centered Broto-Moreau autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation descriptor | 3.37 |
| PaDEL | 2D | ATSC5e | Centered Broto-Moreau autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.71 |
| PaDEL | 2D | ATSC6e | Centered Broto-Moreau autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.23 |
| PaDEL | 2D | ATSC7e | Centered Broto-Moreau autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation descriptor | -4.47 |
| PaDEL | 2D | ATSC8e | Centered Broto-Moreau autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1.04 |
| PaDEL | 2D | ATSC0p | Centered Broto-Moreau autocorrelation - lag 0 / weighted by polarizabilities | Auto correlation descriptor | 13.41 |
| PaDEL | 2D | ATSC1p | Centered Broto-Moreau autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation descriptor | 0.74 |
| PaDEL | 2D | ATSC2p | Centered Broto-Moreau autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation descriptor | 1.3 |
| PaDEL | 2D | ATSC3p | Centered Broto-Moreau autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation descriptor | -4.84 |
| PaDEL | 2D | ATSC4p | Centered Broto-Moreau autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation descriptor | 2.83 |
| PaDEL | 2D | ATSC5p | Centered Broto-Moreau autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation descriptor | 1.94 |
| PaDEL | 2D | ATSC6p | Centered Broto-Moreau autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation descriptor | -7.75 |
| PaDEL | 2D | ATSC7p | Centered Broto-Moreau autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation descriptor | 3.45 |
| PaDEL | 2D | ATSC8p | Centered Broto-Moreau autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation descriptor | -5.93 |
| PaDEL | 2D | ATSC0i | Centered Broto-Moreau autocorrelation - lag 0 / weighted by first ionization potential | Auto correlation descriptor | 75.86 |
| PaDEL | 2D | ATSC1i | Centered Broto-Moreau autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation descriptor | 3.84 |
| PaDEL | 2D | ATSC2i | Centered Broto-Moreau autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation descriptor | 3.14 |
| PaDEL | 2D | ATSC3i | Centered Broto-Moreau autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation descriptor | -27.75 |
| PaDEL | 2D | ATSC4i | Centered Broto-Moreau autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation descriptor | 16.77 |
| PaDEL | 2D | ATSC5i | Centered Broto-Moreau autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation descriptor | 8.14 |
| PaDEL | 2D | ATSC6i | Centered Broto-Moreau autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation descriptor | -42.97 |
| PaDEL | 2D | ATSC7i | Centered Broto-Moreau autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation descriptor | 26.18 |
| PaDEL | 2D | ATSC8i | Centered Broto-Moreau autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation descriptor | -34.98 |
| PaDEL | 2D | ATSC0s | Centered Broto-Moreau autocorrelation - lag 0 / weighted by I-state | Auto correlation descriptor | 85.69 |
| PaDEL | 2D | ATSC1s | Centered Broto-Moreau autocorrelation - lag 1 / weighted by I-state | Auto correlation descriptor | -11.71 |
| PaDEL | 2D | ATSC2s | Centered Broto-Moreau autocorrelation - lag 2 / weighted by I-state | Auto correlation descriptor | 9.76 |
| PaDEL | 2D | ATSC3s | Centered Broto-Moreau autocorrelation - lag 3 / weighted by I-state | Auto correlation descriptor | -27.95 |
| PaDEL | 2D | ATSC4s | Centered Broto-Moreau autocorrelation - lag 4 / weighted by I-state | Auto correlation descriptor | 43.87 |
| PaDEL | 2D | ATSC5s | Centered Broto-Moreau autocorrelation - lag 5 / weighted by I-state | Auto correlation descriptor | -7.79 |
| PaDEL | 2D | ATSC6s | Centered Broto-Moreau autocorrelation - lag 6 / weighted by I-state | Auto correlation descriptor | 5.91 |
| PaDEL | 2D | ATSC7s | Centered Broto-Moreau autocorrelation - lag 7 / weighted by I-state | Auto correlation descriptor | -71.29 |
| PaDEL | 2D | ATSC8s | Centered Broto-Moreau autocorrelation - lag 8 / weighted by I-state | Auto correlation descriptor | 41.01 |
| PaDEL | 2D | AATSC0c | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by charges | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | AATSC1c | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by charges | Auto correlation descriptor | -0.01 |
| PaDEL | 2D | AATSC2c | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by charges | Auto correlation descriptor | -0 |
| PaDEL | 2D | AATSC3c | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by charges | Auto correlation descriptor | -0 |
| PaDEL | 2D | AATSC4c | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by charges | Auto correlation descriptor | 0 |
| PaDEL | 2D | AATSC5c | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by charges | Auto correlation descriptor | -0 |
| PaDEL | 2D | AATSC6c | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by charges | Auto correlation descriptor | 0 |
| PaDEL | 2D | AATSC7c | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by charges | Auto correlation descriptor | -0 |
| PaDEL | 2D | AATSC8c | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by charges | Auto correlation descriptor | 0 |
| PaDEL | 2D | AATSC0m | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by mass | Auto correlation descriptor | 35.04 |
| PaDEL | 2D | AATSC1m | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by mass | Auto correlation descriptor | 0.95 |
| PaDEL | 2D | AATSC2m | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by mass | Auto correlation descriptor | 7.54 |
| PaDEL | 2D | AATSC3m | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by mass | Auto correlation descriptor | -4.96 |
| PaDEL | 2D | AATSC4m | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by mass | Auto correlation descriptor | 4.6 |
| PaDEL | 2D | AATSC5m | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by mass | Auto correlation descriptor | 3.1 |
| PaDEL | 2D | AATSC6m | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by mass | Auto correlation descriptor | -5.01 |
| PaDEL | 2D | AATSC7m | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by mass | Auto correlation descriptor | -9.63 |
| PaDEL | 2D | AATSC8m | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by mass | Auto correlation descriptor | -0.52 |
| PaDEL | 2D | AATSC0v | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by van der Waals volumes | Auto correlation descriptor | 51.49 |
| PaDEL | 2D | AATSC1v | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation descriptor | 2.86 |
| PaDEL | 2D | AATSC2v | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation descriptor | 7.62 |
| PaDEL | 2D | AATSC3v | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation descriptor | -7.42 |
| PaDEL | 2D | AATSC4v | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation descriptor | 4.1 |
| PaDEL | 2D | AATSC5v | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation descriptor | 4.63 |
| PaDEL | 2D | AATSC6v | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation descriptor | -11.05 |
| PaDEL | 2D | AATSC7v | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation descriptor | -2.6 |
| PaDEL | 2D | AATSC8v | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation descriptor | -6.29 |
| PaDEL | 2D | AATSC0e | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.08 |
| PaDEL | 2D | AATSC1e | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.01 |
| PaDEL | 2D | AATSC2e | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0 |
| PaDEL | 2D | AATSC3e | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.01 |
| PaDEL | 2D | AATSC4e | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | AATSC5e | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0 |
| PaDEL | 2D | AATSC6e | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0 |
| PaDEL | 2D | AATSC7e | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.03 |
| PaDEL | 2D | AATSC8e | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.01 |
| PaDEL | 2D | AATSC0p | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by polarizabilities | Auto correlation descriptor | 0.24 |
| PaDEL | 2D | AATSC1p | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation descriptor | 0.01 |
| PaDEL | 2D | AATSC2p | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation descriptor | 0.01 |
| PaDEL | 2D | AATSC3p | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation descriptor | -0.04 |
| PaDEL | 2D | AATSC4p | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | AATSC5p | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation descriptor | 0.01 |
| PaDEL | 2D | AATSC6p | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation descriptor | -0.05 |
| PaDEL | 2D | AATSC7p | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | AATSC8p | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation descriptor | -0.04 |
| PaDEL | 2D | AATSC0i | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by first ionization potential | Auto correlation descriptor | 1.35 |
| PaDEL | 2D | AATSC1i | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation descriptor | 0.07 |
| PaDEL | 2D | AATSC2i | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation descriptor | 0.03 |
| PaDEL | 2D | AATSC3i | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation descriptor | -0.21 |
| PaDEL | 2D | AATSC4i | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation descriptor | 0.1 |
| PaDEL | 2D | AATSC5i | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation descriptor | 0.05 |
| PaDEL | 2D | AATSC6i | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation descriptor | -0.3 |
| PaDEL | 2D | AATSC7i | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation descriptor | 0.16 |
| PaDEL | 2D | AATSC8i | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation descriptor | -0.24 |
| PaDEL | 2D | AATSC0s | Average centered Broto-Moreau autocorrelation - lag 0 / weighted by I-state | Auto correlation descriptor | 1.53 |
| PaDEL | 2D | AATSC1s | Average centered Broto-Moreau autocorrelation - lag 1 / weighted by I-state | Auto correlation descriptor | -0.2 |
| PaDEL | 2D | AATSC2s | Average centered Broto-Moreau autocorrelation - lag 2 / weighted by I-state | Auto correlation descriptor | 0.09 |
| PaDEL | 2D | AATSC3s | Average centered Broto-Moreau autocorrelation - lag 3 / weighted by I-state | Auto correlation descriptor | -0.21 |
| PaDEL | 2D | AATSC4s | Average centered Broto-Moreau autocorrelation - lag 4 / weighted by I-state | Auto correlation descriptor | 0.27 |
| PaDEL | 2D | AATSC5s | Average centered Broto-Moreau autocorrelation - lag 5 / weighted by I-state | Auto correlation descriptor | -0.05 |
| PaDEL | 2D | AATSC6s | Average centered Broto-Moreau autocorrelation - lag 6 / weighted by I-state | Auto correlation descriptor | 0.04 |
| PaDEL | 2D | AATSC7s | Average centered Broto-Moreau autocorrelation - lag 7 / weighted by I-state | Auto correlation descriptor | -0.44 |
| PaDEL | 2D | AATSC8s | Average centered Broto-Moreau autocorrelation - lag 8 / weighted by I-state | Auto correlation descriptor | 0.28 |
| PaDEL | 2D | MATS1c | Moran autocorrelation - lag 1 / weighted by charges | Auto correlation descriptor | -0.39 |
| PaDEL | 2D | MATS2c | Moran autocorrelation - lag 2 / weighted by charges | Auto correlation descriptor | -0.02 |
| PaDEL | 2D | MATS3c | Moran autocorrelation - lag 3 / weighted by charges | Auto correlation descriptor | -0.2 |
| PaDEL | 2D | MATS4c | Moran autocorrelation - lag 4 / weighted by charges | Auto correlation descriptor | 0.23 |
| PaDEL | 2D | MATS5c | Moran autocorrelation - lag 5 / weighted by charges | Auto correlation descriptor | -0.15 |
| PaDEL | 2D | MATS6c | Moran autocorrelation - lag 6 / weighted by charges | Auto correlation descriptor | 0.19 |
| PaDEL | 2D | MATS7c | Moran autocorrelation - lag 7 / weighted by charges | Auto correlation descriptor | -0.19 |
| PaDEL | 2D | MATS8c | Moran autocorrelation - lag 8 / weighted by charges | Auto correlation descriptor | 0.14 |
| PaDEL | 2D | MATS1m | Moran autocorrelation - lag 1 / weighted by mass | Auto correlation descriptor | 0.03 |
| PaDEL | 2D | MATS2m | Moran autocorrelation - lag 2 / weighted by mass | Auto correlation descriptor | 0.22 |
| PaDEL | 2D | MATS3m | Moran autocorrelation - lag 3 / weighted by mass | Auto correlation descriptor | -0.14 |
| PaDEL | 2D | MATS4m | Moran autocorrelation - lag 4 / weighted by mass | Auto correlation descriptor | 0.13 |
| PaDEL | 2D | MATS5m | Moran autocorrelation - lag 5 / weighted by mass | Auto correlation descriptor | 0.09 |
| PaDEL | 2D | MATS6m | Moran autocorrelation - lag 6 / weighted by mass | Auto correlation descriptor | -0.14 |
| PaDEL | 2D | MATS7m | Moran autocorrelation - lag 7 / weighted by mass | Auto correlation descriptor | -0.27 |
| PaDEL | 2D | MATS8m | Moran autocorrelation - lag 8 / weighted by mass | Auto correlation descriptor | -0.01 |
| PaDEL | 2D | MATS1v | Moran autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation descriptor | 0.06 |
| PaDEL | 2D | MATS2v | Moran autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation descriptor | 0.15 |
| PaDEL | 2D | MATS3v | Moran autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation descriptor | -0.14 |
| PaDEL | 2D | MATS4v | Moran autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation descriptor | 0.08 |
| PaDEL | 2D | MATS5v | Moran autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation descriptor | 0.09 |
| PaDEL | 2D | MATS6v | Moran autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation descriptor | -0.21 |
| PaDEL | 2D | MATS7v | Moran autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation descriptor | -0.05 |
| PaDEL | 2D | MATS8v | Moran autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation descriptor | -0.12 |
| PaDEL | 2D | MATS1e | Moran autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.06 |
| PaDEL | 2D | MATS2e | Moran autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | MATS3e | Moran autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.16 |
| PaDEL | 2D | MATS4e | Moran autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.25 |
| PaDEL | 2D | MATS5e | Moran autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.06 |
| PaDEL | 2D | MATS6e | Moran autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | MATS7e | Moran autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation descriptor | -0.33 |
| PaDEL | 2D | MATS8e | Moran autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.08 |
| PaDEL | 2D | MATS1p | Moran autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation descriptor | 0.05 |
| PaDEL | 2D | MATS2p | Moran autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation descriptor | 0.05 |
| PaDEL | 2D | MATS3p | Moran autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation descriptor | -0.15 |
| PaDEL | 2D | MATS4p | Moran autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation descriptor | 0.07 |
| PaDEL | 2D | MATS5p | Moran autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation descriptor | 0.05 |
| PaDEL | 2D | MATS6p | Moran autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation descriptor | -0.22 |
| PaDEL | 2D | MATS7p | Moran autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation descriptor | 0.09 |
| PaDEL | 2D | MATS8p | Moran autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation descriptor | -0.17 |
| PaDEL | 2D | MATS1i | Moran autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation descriptor | 0.05 |
| PaDEL | 2D | MATS2i | Moran autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation descriptor | 0.02 |
| PaDEL | 2D | MATS3i | Moran autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation descriptor | -0.15 |
| PaDEL | 2D | MATS4i | Moran autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation descriptor | 0.08 |
| PaDEL | 2D | MATS5i | Moran autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation descriptor | 0.04 |
| PaDEL | 2D | MATS6i | Moran autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation descriptor | -0.22 |
| PaDEL | 2D | MATS7i | Moran autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation descriptor | 0.12 |
| PaDEL | 2D | MATS8i | Moran autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation descriptor | -0.18 |
| PaDEL | 2D | MATS1s | Moran autocorrelation - lag 1 / weighted by I-state | Auto correlation descriptor | -0.13 |
| PaDEL | 2D | MATS2s | Moran autocorrelation - lag 2 / weighted by I-state | Auto correlation descriptor | 0.06 |
| PaDEL | 2D | MATS3s | Moran autocorrelation - lag 3 / weighted by I-state | Auto correlation descriptor | -0.14 |
| PaDEL | 2D | MATS4s | Moran autocorrelation - lag 4 / weighted by I-state | Auto correlation descriptor | 0.18 |
| PaDEL | 2D | MATS5s | Moran autocorrelation - lag 5 / weighted by I-state | Auto correlation descriptor | -0.03 |
| PaDEL | 2D | MATS6s | Moran autocorrelation - lag 6 / weighted by I-state | Auto correlation descriptor | 0.03 |
| PaDEL | 2D | MATS7s | Moran autocorrelation - lag 7 / weighted by I-state | Auto correlation descriptor | -0.29 |
| PaDEL | 2D | MATS8s | Moran autocorrelation - lag 8 / weighted by I-state | Auto correlation descriptor | 0.18 |
| PaDEL | 2D | GATS1c | Geary autocorrelation - lag 1 / weighted by charges | Auto correlation descriptor | 1.29 |
| PaDEL | 2D | GATS2c | Geary autocorrelation - lag 2 / weighted by charges | Auto correlation descriptor | 0.9 |
| PaDEL | 2D | GATS3c | Geary autocorrelation - lag 3 / weighted by charges | Auto correlation descriptor | 1.4 |
| PaDEL | 2D | GATS4c | Geary autocorrelation - lag 4 / weighted by charges | Auto correlation descriptor | 0.89 |
| PaDEL | 2D | GATS5c | Geary autocorrelation - lag 5 / weighted by charges | Auto correlation descriptor | 1.15 |
| PaDEL | 2D | GATS6c | Geary autocorrelation - lag 6 / weighted by charges | Auto correlation descriptor | 1.01 |
| PaDEL | 2D | GATS7c | Geary autocorrelation - lag 7 / weighted by charges | Auto correlation descriptor | 1.58 |
| PaDEL | 2D | GATS8c | Geary autocorrelation - lag 8 / weighted by charges | Auto correlation descriptor | 0.73 |
| PaDEL | 2D | GATS1m | Geary autocorrelation - lag 1 / weighted by mass | Auto correlation descriptor | 0.82 |
| PaDEL | 2D | GATS2m | Geary autocorrelation - lag 2 / weighted by mass | Auto correlation descriptor | 0.69 |
| PaDEL | 2D | GATS3m | Geary autocorrelation - lag 3 / weighted by mass | Auto correlation descriptor | 1.1 |
| PaDEL | 2D | GATS4m | Geary autocorrelation - lag 4 / weighted by mass | Auto correlation descriptor | 0.87 |
| PaDEL | 2D | GATS5m | Geary autocorrelation - lag 5 / weighted by mass | Auto correlation descriptor | 0.87 |
| PaDEL | 2D | GATS6m | Geary autocorrelation - lag 6 / weighted by mass | Auto correlation descriptor | 1.11 |
| PaDEL | 2D | GATS7m | Geary autocorrelation - lag 7 / weighted by mass | Auto correlation descriptor | 1.32 |
| PaDEL | 2D | GATS8m | Geary autocorrelation - lag 8 / weighted by mass | Auto correlation descriptor | 0.96 |
| PaDEL | 2D | GATS1v | Geary autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation descriptor | 0.94 |
| PaDEL | 2D | GATS2v | Geary autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation descriptor | 0.85 |
| PaDEL | 2D | GATS3v | Geary autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation descriptor | 1.09 |
| PaDEL | 2D | GATS4v | Geary autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation descriptor | 0.89 |
| PaDEL | 2D | GATS5v | Geary autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation descriptor | 0.9 |
| PaDEL | 2D | GATS6v | Geary autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation descriptor | 1.17 |
| PaDEL | 2D | GATS7v | Geary autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation descriptor | 0.98 |
| PaDEL | 2D | GATS8v | Geary autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation descriptor | 1.13 |
| PaDEL | 2D | GATS1e | Geary autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.85 |
| PaDEL | 2D | GATS2e | Geary autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.81 |
| PaDEL | 2D | GATS3e | Geary autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1.37 |
| PaDEL | 2D | GATS4e | Geary autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.89 |
| PaDEL | 2D | GATS5e | Geary autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1.01 |
| PaDEL | 2D | GATS6e | Geary autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1.16 |
| PaDEL | 2D | GATS7e | Geary autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation descriptor | 1.77 |
| PaDEL | 2D | GATS8e | Geary autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation descriptor | 0.65 |
| PaDEL | 2D | GATS1p | Geary autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation descriptor | 1.02 |
| PaDEL | 2D | GATS2p | Geary autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation descriptor | 0.99 |
| PaDEL | 2D | GATS3p | Geary autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation descriptor | 1.15 |
| PaDEL | 2D | GATS4p | Geary autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation descriptor | 0.9 |
| PaDEL | 2D | GATS5p | Geary autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation descriptor | 0.94 |
| PaDEL | 2D | GATS6p | Geary autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation descriptor | 1.22 |
| PaDEL | 2D | GATS7p | Geary autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation descriptor | 0.86 |
| PaDEL | 2D | GATS8p | Geary autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation descriptor | 1.17 |
| PaDEL | 2D | GATS1i | Geary autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation descriptor | 1.05 |
| PaDEL | 2D | GATS2i | Geary autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation descriptor | 1.02 |
| PaDEL | 2D | GATS3i | Geary autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation descriptor | 1.18 |
| PaDEL | 2D | GATS4i | Geary autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation descriptor | 0.91 |
| PaDEL | 2D | GATS5i | Geary autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation descriptor | 0.96 |
| PaDEL | 2D | GATS6i | Geary autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation descriptor | 1.23 |
| PaDEL | 2D | GATS7i | Geary autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation descriptor | 0.85 |
| PaDEL | 2D | GATS8i | Geary autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation descriptor | 1.16 |
| PaDEL | 2D | GATS1s | Geary autocorrelation - lag 1 / weighted by I-state | Auto correlation descriptor | 0.85 |
| PaDEL | 2D | GATS2s | Geary autocorrelation - lag 2 / weighted by I-state | Auto correlation descriptor | 0.57 |
| PaDEL | 2D | GATS3s | Geary autocorrelation - lag 3 / weighted by I-state | Auto correlation descriptor | 1.08 |
| PaDEL | 2D | GATS4s | Geary autocorrelation - lag 4 / weighted by I-state | Auto correlation descriptor | 0.83 |
| PaDEL | 2D | GATS5s | Geary autocorrelation - lag 5 / weighted by I-state | Auto correlation descriptor | 0.95 |
| PaDEL | 2D | GATS6s | Geary autocorrelation - lag 6 / weighted by I-state | Auto correlation descriptor | 1.26 |
| PaDEL | 2D | GATS7s | Geary autocorrelation - lag 7 / weighted by I-state | Auto correlation descriptor | 1.65 |
| PaDEL | 2D | GATS8s | Geary autocorrelation - lag 8 / weighted by I-state | Auto correlation descriptor | 0.63 |
| PaDEL | 2D | SpAbs_DzZ | Graph energy from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 254.36 |
| PaDEL | 2D | SpMax_DzZ | Leading eigenvalue from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 126.82 |
| PaDEL | 2D | SpDiam_DzZ | Spectral diameter from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 178.84 |
| PaDEL | 2D | SpAD_DzZ | Spectral absolute deviation from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 255.18 |
| PaDEL | 2D | SpMAD_DzZ | Spectral mean absolute deviation from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 8.51 |
| PaDEL | 2D | EE_DzZ | Estrada-like index from Barysz matrix / weighted by atomic number (ln(1+x)) | Barysz Matrix descriptor | 126.82 |
| PaDEL | 2D | SM1_DzZ | Spectral moment of order 1 from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 1.25 |
| PaDEL | 2D | VE1_DzZ | Coefficient sum of the last eigenvector from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 0.15 |
| PaDEL | 2D | VE2_DzZ | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 0 |
| PaDEL | 2D | VE3_DzZ | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | -5.75 |
| PaDEL | 2D | VR1_DzZ | Randic-like eigenvector-based index from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 694.35 |
| PaDEL | 2D | VR2_DzZ | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 23.14 |
| PaDEL | 2D | VR3_DzZ | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by atomic number | Barysz Matrix descriptor | 19.63 |
| PaDEL | 2D | SpAbs_Dzm | Graph energy from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 254.41 |
| PaDEL | 2D | SpMax_Dzm | Leading eigenvalue from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 126.84 |
| PaDEL | 2D | SpDiam_Dzm | Spectral diameter from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 178.87 |
| PaDEL | 2D | SpAD_Dzm | Spectral absolute deviation from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 255.22 |
| PaDEL | 2D | SpMAD_Dzm | Spectral mean absolute deviation from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 8.51 |
| PaDEL | 2D | EE_Dzm | Estrada-like index from Barysz matrix / weighted by mass (ln(1+x)) | Barysz Matrix descriptor | 126.84 |
| PaDEL | 2D | SM1_Dzm | Spectral moment of order 1 from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 1.25 |
| PaDEL | 2D | VE1_Dzm | Coefficient sum of the last eigenvector from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 0.15 |
| PaDEL | 2D | VE2_Dzm | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 0 |
| PaDEL | 2D | VE3_Dzm | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by mass | Barysz Matrix descriptor | -5.75 |
| PaDEL | 2D | VR1_Dzm | Randic-like eigenvector-based index from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 709.27 |
| PaDEL | 2D | VR2_Dzm | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 23.64 |
| PaDEL | 2D | VR3_Dzm | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by mass | Barysz Matrix descriptor | 19.69 |
| PaDEL | 2D | SpAbs_Dzv | Graph energy from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 301.76 |
| PaDEL | 2D | SpMax_Dzv | Leading eigenvalue from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 148.12 |
| PaDEL | 2D | SpDiam_Dzv | Spectral diameter from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 210.54 |
| PaDEL | 2D | SpAD_Dzv | Spectral absolute deviation from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 300.57 |
| PaDEL | 2D | SpMAD_Dzv | Spectral mean absolute deviation from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 10.02 |
| PaDEL | 2D | EE_Dzv | Estrada-like index from Barysz matrix / weighted by van der Waals volumes (ln(1+x)) | Barysz Matrix descriptor | 148.12 |
| PaDEL | 2D | SM1_Dzv | Spectral moment of order 1 from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | -2 |
| PaDEL | 2D | VE1_Dzv | Coefficient sum of the last eigenvector from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 0.17 |
| PaDEL | 2D | VE2_Dzv | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by van der Waals volum | Barysz Matrix descriptor | 0.01 |
| PaDEL | 2D | VE3_Dzv | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by van der Waals v | Barysz Matrix descriptor | -5.28 |
| PaDEL | 2D | VR1_Dzv | Randic-like eigenvector-based index from Barysz matrix / weighted by van der Waals volumes | Barysz Matrix descriptor | 316.31 |
| PaDEL | 2D | VR2_Dzv | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by van der Waals volume | Barysz Matrix descriptor | 10.54 |
| PaDEL | 2D | VR3_Dzv | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by van der Waals volum | Barysz Matrix descriptor | 17.27 |
| PaDEL | 2D | SpAbs_Dze | Graph energy from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 254.46 |
| PaDEL | 2D | SpMax_Dze | Leading eigenvalue from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 126.87 |
| PaDEL | 2D | SpDiam_Dze | Spectral diameter from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 178.91 |
| PaDEL | 2D | SpAD_Dze | Spectral absolute deviation from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 255.27 |
| PaDEL | 2D | SpMAD_Dze | Spectral mean absolute deviation from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 8.51 |
| PaDEL | 2D | EE_Dze | Estrada-like index from Barysz matrix / weighted by Sanderson electronegativities (ln(1+x)) | Barysz Matrix descriptor | 126.87 |
| PaDEL | 2D | SM1_Dze | Spectral moment of order 1 from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 1.24 |
| PaDEL | 2D | VE1_Dze | Coefficient sum of the last eigenvector from Barysz matrix / weighted by Sanderson electronegativiti | Barysz Matrix descriptor | 0.15 |
| PaDEL | 2D | VE2_Dze | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by Sanderson electrone | Barysz Matrix descriptor | 0 |
| PaDEL | 2D | VE3_Dze | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by Sanderson elect | Barysz Matrix descriptor | -5.75 |
| PaDEL | 2D | VR1_Dze | Randic-like eigenvector-based index from Barysz matrix / weighted by Sanderson electronegativities | Barysz Matrix descriptor | 726.85 |
| PaDEL | 2D | VR2_Dze | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by Sanderson electroneg | Barysz Matrix descriptor | 24.23 |
| PaDEL | 2D | VR3_Dze | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by Sanderson electrone | Barysz Matrix descriptor | 19.77 |
| PaDEL | 2D | SpAbs_Dzp | Graph energy from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 359.47 |
| PaDEL | 2D | SpMax_Dzp | Leading eigenvalue from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 171.14 |
| PaDEL | 2D | SpDiam_Dzp | Spectral diameter from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 245.65 |
| PaDEL | 2D | SpAD_Dzp | Spectral absolute deviation from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 356.58 |
| PaDEL | 2D | SpMAD_Dzp | Spectral mean absolute deviation from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 11.89 |
| PaDEL | 2D | EE_Dzp | Estrada-like index from Barysz matrix / weighted by polarizabilities (ln(1+x)) | Barysz Matrix descriptor | 171.14 |
| PaDEL | 2D | SM1_Dzp | Spectral moment of order 1 from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | -5.41 |
| PaDEL | 2D | VE1_Dzp | Coefficient sum of the last eigenvector from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 0.17 |
| PaDEL | 2D | VE2_Dzp | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 0.01 |
| PaDEL | 2D | VE3_Dzp | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by polarizabilitie | Barysz Matrix descriptor | -5.3 |
| PaDEL | 2D | VR1_Dzp | Randic-like eigenvector-based index from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 313.05 |
| PaDEL | 2D | VR2_Dzp | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 10.44 |
| PaDEL | 2D | VR3_Dzp | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by polarizabilities | Barysz Matrix descriptor | 17.24 |
| PaDEL | 2D | SpAbs_Dzi | Graph energy from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 259.36 |
| PaDEL | 2D | SpMax_Dzi | Leading eigenvalue from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 129.3 |
| PaDEL | 2D | SpDiam_Dzi | Spectral diameter from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 182.48 |
| PaDEL | 2D | SpAD_Dzi | Spectral absolute deviation from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 259.98 |
| PaDEL | 2D | SpMAD_Dzi | Spectral mean absolute deviation from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 8.67 |
| PaDEL | 2D | EE_Dzi | Estrada-like index from Barysz matrix / weighted by first ionization potential (ln(1+x)) | Barysz Matrix descriptor | 129.3 |
| PaDEL | 2D | SM1_Dzi | Spectral moment of order 1 from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 0.87 |
| PaDEL | 2D | VE1_Dzi | Coefficient sum of the last eigenvector from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 0.15 |
| PaDEL | 2D | VE2_Dzi | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by first ionization po | Barysz Matrix descriptor | 0.01 |
| PaDEL | 2D | VE3_Dzi | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by first ionizatio | Barysz Matrix descriptor | -5.65 |
| PaDEL | 2D | VR1_Dzi | Randic-like eigenvector-based index from Barysz matrix / weighted by first ionization potential | Barysz Matrix descriptor | 442.62 |
| PaDEL | 2D | VR2_Dzi | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by first ionization pot | Barysz Matrix descriptor | 14.75 |
| PaDEL | 2D | VR3_Dzi | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by first ionization po | Barysz Matrix descriptor | 18.28 |
| PaDEL | 2D | SpAbs_Dzs | Graph energy from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 270.52 |
| PaDEL | 2D | SpMax_Dzs | Leading eigenvalue from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 133.92 |
| PaDEL | 2D | SpDiam_Dzs | Spectral diameter from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 190.55 |
| PaDEL | 2D | SpAD_Dzs | Spectral absolute deviation from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 271.92 |
| PaDEL | 2D | SpMAD_Dzs | Spectral mean absolute deviation from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 9.06 |
| PaDEL | 2D | EE_Dzs | Estrada-like index from Barysz matrix / weighted by I-state (ln(1+x)) | Barysz Matrix descriptor | 133.92 |
| PaDEL | 2D | SM1_Dzs | Spectral moment of order 1 from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 2.69 |
| PaDEL | 2D | VE1_Dzs | Coefficient sum of the last eigenvector from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 0.17 |
| PaDEL | 2D | VE2_Dzs | Average coefficient sum of the last eigenvector from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 0.01 |
| PaDEL | 2D | VE3_Dzs | Logarithmic coefficient sum of the last eigenvector from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | -5.4 |
| PaDEL | 2D | VR1_Dzs | Randic-like eigenvector-based index from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 439.87 |
| PaDEL | 2D | VR2_Dzs | Normalized Randic-like eigenvector-based index from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 14.66 |
| PaDEL | 2D | VR3_Dzs | Logarithmic Randic-like eigenvector-based index from Barysz matrix / weighted by I-state | Barysz Matrix descriptor | 18.26 |
| PaDEL | 2D | nBase | Number of basic groups. The list of basic groups is defined by this SMARTS "[$([NH2]-[CX4])]", "[$([ | Basic Group Count descriptor | 0 |
| PaDEL | 2D | BCUTw_1l | nhigh lowest atom weighted BCUTS | BCUT descriptor | 11.89 |
| PaDEL | 2D | BCUTw_1h | nlow highest atom weighted BCUTS | BCUT descriptor | 16 |
| PaDEL | 2D | BCUTc_1l | nhigh lowest partial charge weighted BCUTS | BCUT descriptor | -0.39 |
| PaDEL | 2D | BCUTc_1h | nlow highest partial charge weighted BCUTS | BCUT descriptor | 0.15 |
| PaDEL | 2D | BCUTp_1l | nhigh lowest polarizability weighted BCUTS | BCUT descriptor | 4.81 |
| PaDEL | 2D | BCUTp_1h | nlow highest polarizability weighted BCUTS | BCUT descriptor | 11.11 |
| PaDEL | 2D | nBonds | Number of bonds (excluding bonds with hydrogen) | PaDEL Bond Count descriptor | 33 |
| PaDEL | 2D | nBonds2 | Total number of bonds (including bonds to hydrogens) | PaDEL Bond Count descriptor | 59 |
| PaDEL | 2D | nBondsS | Number of single bonds (including bonds with hydrogen) | PaDEL Bond Count descriptor | 56 |
| PaDEL | 2D | nBondsS2 | Total number of single bonds (including bonds to hydrogens, excluding aromatic bonds) | PaDEL Bond Count descriptor | 44 |
| PaDEL | 2D | nBondsS3 | Total number of single bonds (excluding bonds to hydrogens and aromatic bonds) | PaDEL Bond Count descriptor | 18 |
| PaDEL | 2D | nBondsD | Number of double bonds | PaDEL Bond Count descriptor | 3 |
| PaDEL | 2D | nBondsD2 | Total number of double bonds (excluding bonds to aromatic bonds) | PaDEL Bond Count descriptor | 3 |
| PaDEL | 2D | nBondsT | Number of triple bonds | PaDEL Bond Count descriptor | 0 |
| PaDEL | 2D | nBondsQ | Number of quadruple bonds | PaDEL Bond Count descriptor | 0 |
| PaDEL | 2D | nBondsM | Total number of bonds that have bond order greater than one (aromatic bonds have bond order 1.5). | PaDEL Bond Count descriptor | 15 |
| PaDEL | 2D | bpol | Sum of the absolute value of the difference between atomic polarizabilities of all bonded atoms in t | BPol descriptor | 33.21 |
| PaDEL | 2D | SpMax1_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative mass | Burden Modified Eigen values descriptor | 4.01 |
| PaDEL | 2D | SpMax2_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative mass | Burden Modified Eigen values descriptor | 3.81 |
| PaDEL | 2D | SpMax3_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative mass | Burden Modified Eigen values descriptor | 3.57 |
| PaDEL | 2D | SpMax4_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative mass | Burden Modified Eigen values descriptor | 3.52 |
| PaDEL | 2D | SpMax5_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative mass | Burden Modified Eigen values descriptor | 3.27 |
| PaDEL | 2D | SpMax6_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative mass | Burden Modified Eigen values descriptor | 3.04 |
| PaDEL | 2D | SpMax7_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative mass | Burden Modified Eigen values descriptor | 2.93 |
| PaDEL | 2D | SpMax8_Bhm | Largest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative mass | Burden Modified Eigen values descriptor | 2.67 |
| PaDEL | 2D | SpMin1_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative mass | Burden Modified Eigen values descriptor | 2.04 |
| PaDEL | 2D | SpMin2_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.94 |
| PaDEL | 2D | SpMin3_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.83 |
| PaDEL | 2D | SpMin4_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.74 |
| PaDEL | 2D | SpMin5_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.54 |
| PaDEL | 2D | SpMin6_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.38 |
| PaDEL | 2D | SpMin7_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.25 |
| PaDEL | 2D | SpMin8_Bhm | Smallest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative mass | Burden Modified Eigen values descriptor | 1.25 |
| PaDEL | 2D | SpMax1_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 3.97 |
| PaDEL | 2D | SpMax2_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 3.8 |
| PaDEL | 2D | SpMax3_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 3.55 |
| PaDEL | 2D | SpMax4_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 3.51 |
| PaDEL | 2D | SpMax5_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 3.24 |
| PaDEL | 2D | SpMax6_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 2.99 |
| PaDEL | 2D | SpMax7_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 2.87 |
| PaDEL | 2D | SpMax8_Bhv | Largest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative van der Waals vol | Burden Modified Eigen values descriptor | 2.65 |
| PaDEL | 2D | SpMin1_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 2.07 |
| PaDEL | 2D | SpMin2_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.93 |
| PaDEL | 2D | SpMin3_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.79 |
| PaDEL | 2D | SpMin4_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.73 |
| PaDEL | 2D | SpMin5_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.51 |
| PaDEL | 2D | SpMin6_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.32 |
| PaDEL | 2D | SpMin7_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.16 |
| PaDEL | 2D | SpMin8_Bhv | Smallest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative van der Waals vo | Burden Modified Eigen values descriptor | 1.13 |
| PaDEL | 2D | SpMax1_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 4.03 |
| PaDEL | 2D | SpMax2_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 3.86 |
| PaDEL | 2D | SpMax3_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 3.64 |
| PaDEL | 2D | SpMax4_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 3.62 |
| PaDEL | 2D | SpMax5_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 3.36 |
| PaDEL | 2D | SpMax6_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 3.13 |
| PaDEL | 2D | SpMax7_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 3.01 |
| PaDEL | 2D | SpMax8_Bhe | Largest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative Sanderson electro | Burden Modified Eigen values descriptor | 2.83 |
| PaDEL | 2D | SpMin1_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 1.99 |
| PaDEL | 2D | SpMin2_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 1.85 |
| PaDEL | 2D | SpMin3_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 1.64 |
| PaDEL | 2D | SpMin4_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 1.59 |
| PaDEL | 2D | SpMin5_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 1.34 |
| PaDEL | 2D | SpMin6_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 1.12 |
| PaDEL | 2D | SpMin7_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 0.96 |
| PaDEL | 2D | SpMin8_Bhe | Smallest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative Sanderson electr | Burden Modified Eigen values descriptor | 0.8 |
| PaDEL | 2D | SpMax1_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 3.96 |
| PaDEL | 2D | SpMax2_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 3.8 |
| PaDEL | 2D | SpMax3_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 3.56 |
| PaDEL | 2D | SpMax4_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 3.51 |
| PaDEL | 2D | SpMax5_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 3.24 |
| PaDEL | 2D | SpMax6_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 2.99 |
| PaDEL | 2D | SpMax7_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 2.86 |
| PaDEL | 2D | SpMax8_Bhp | Largest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 2.67 |
| PaDEL | 2D | SpMin1_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 2.08 |
| PaDEL | 2D | SpMin2_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.93 |
| PaDEL | 2D | SpMin3_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.76 |
| PaDEL | 2D | SpMin4_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.73 |
| PaDEL | 2D | SpMin5_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.49 |
| PaDEL | 2D | SpMin6_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.29 |
| PaDEL | 2D | SpMin7_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.16 |
| PaDEL | 2D | SpMin8_Bhp | Smallest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative polarizabilities | Burden Modified Eigen values descriptor | 1.06 |
| PaDEL | 2D | SpMax1_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 4.03 |
| PaDEL | 2D | SpMax2_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 3.88 |
| PaDEL | 2D | SpMax3_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 3.68 |
| PaDEL | 2D | SpMax4_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 3.64 |
| PaDEL | 2D | SpMax5_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 3.39 |
| PaDEL | 2D | SpMax6_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 3.17 |
| PaDEL | 2D | SpMax7_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 3.03 |
| PaDEL | 2D | SpMax8_Bhi | Largest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 2.88 |
| PaDEL | 2D | SpMin1_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 1.98 |
| PaDEL | 2D | SpMin2_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 1.83 |
| PaDEL | 2D | SpMin3_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 1.61 |
| PaDEL | 2D | SpMin4_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 1.56 |
| PaDEL | 2D | SpMin5_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 1.31 |
| PaDEL | 2D | SpMin6_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 1.07 |
| PaDEL | 2D | SpMin7_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 0.93 |
| PaDEL | 2D | SpMin8_Bhi | Smallest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative first ionization | Burden Modified Eigen values descriptor | 0.74 |
| PaDEL | 2D | SpMax1_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative I-state | Burden Modified Eigen values descriptor | 4.71 |
| PaDEL | 2D | SpMax2_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative I-state | Burden Modified Eigen values descriptor | 4.23 |
| PaDEL | 2D | SpMax3_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative I-state | Burden Modified Eigen values descriptor | 4.2 |
| PaDEL | 2D | SpMax4_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative I-state | Burden Modified Eigen values descriptor | 3.86 |
| PaDEL | 2D | SpMax5_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative I-state | Burden Modified Eigen values descriptor | 3.68 |
| PaDEL | 2D | SpMax6_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative I-state | Burden Modified Eigen values descriptor | 3.6 |
| PaDEL | 2D | SpMax7_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative I-state | Burden Modified Eigen values descriptor | 3.41 |
| PaDEL | 2D | SpMax8_Bhs | Largest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative I-state | Burden Modified Eigen values descriptor | 3.14 |
| PaDEL | 2D | SpMin1_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 1 / weighted by relative I-state | Burden Modified Eigen values descriptor | 2 |
| PaDEL | 2D | SpMin2_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 2 / weighted by relative I-state | Burden Modified Eigen values descriptor | 1.85 |
| PaDEL | 2D | SpMin3_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 3 / weighted by relative I-state | Burden Modified Eigen values descriptor | 1.69 |
| PaDEL | 2D | SpMin4_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 4 / weighted by relative I-state | Burden Modified Eigen values descriptor | 1.61 |
| PaDEL | 2D | SpMin5_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 5 / weighted by relative I-state | Burden Modified Eigen values descriptor | 1.42 |
| PaDEL | 2D | SpMin6_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 6 / weighted by relative I-state | Burden Modified Eigen values descriptor | 1.19 |
| PaDEL | 2D | SpMin7_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 7 / weighted by relative I-state | Burden Modified Eigen values descriptor | 1.01 |
| PaDEL | 2D | SpMin8_Bhs | Smallest absolute eigenvalue of Burden modified matrix - n 8 / weighted by relative I-state | Burden Modified Eigen values descriptor | 0.91 |
| PaDEL | 2D | C1SP1 | Triply bound carbon bound to one other carbon | PaDEL Carbon Types descriptor | 0 |
| PaDEL | 2D | C2SP1 | Triply bound carbon bound to two other carbons | PaDEL Carbon Types descriptor | 0 |
| PaDEL | 2D | C1SP2 | Doubly bound carbon bound to one other carbon | PaDEL Carbon Types descriptor | 0 |
| PaDEL | 2D | C2SP2 | Doubly bound carbon bound to two other carbons | PaDEL Carbon Types descriptor | 12 |
| PaDEL | 2D | C3SP2 | Doubly bound carbon bound to three other carbons | PaDEL Carbon Types descriptor | 5 |
| PaDEL | 2D | C1SP3 | Singly bound carbon bound to one other carbon | PaDEL Carbon Types descriptor | 4 |
| PaDEL | 2D | C2SP3 | Singly bound carbon bound to two other carbons | PaDEL Carbon Types descriptor | 3 |
| PaDEL | 2D | C3SP3 | Singly bound carbon bound to three other carbons | PaDEL Carbon Types descriptor | 1 |
| PaDEL | 2D | C4SP3 | Singly bound carbon bound to four other carbons | PaDEL Carbon Types descriptor | 0 |
| PaDEL | 2D | SCH_3 | Simple chain, order 3 | ChiChain descriptor | 0 |
| PaDEL | 2D | SCH_4 | Simple chain, order 4 | ChiChain descriptor | 0 |
| PaDEL | 2D | SCH_5 | Simple chain, order 5 | ChiChain descriptor | 0 |
| PaDEL | 2D | SCH_6 | Simple chain, order 6 | ChiChain descriptor | 0.23 |
| PaDEL | 2D | SCH_7 | Simple chain, order 7 | ChiChain descriptor | 0.63 |
| PaDEL | 2D | VCH_3 | Valence chain, order 3 | ChiChain descriptor | 0 |
| PaDEL | 2D | VCH_4 | Valence chain, order 4 | ChiChain descriptor | 0 |
| PaDEL | 2D | VCH_5 | Valence chain, order 5 | ChiChain descriptor | 0 |
| PaDEL | 2D | VCH_6 | Valence chain, order 6 | ChiChain descriptor | 0.08 |
| PaDEL | 2D | VCH_7 | Valence chain, order 7 | ChiChain descriptor | 0.17 |
| PaDEL | 2D | SC_3 | Simple cluster, order 3 | ChiCluster descriptor | 3.32 |
| PaDEL | 2D | SC_4 | Simple cluster, order 4 | ChiCluster descriptor | 0.25 |
| PaDEL | 2D | SC_5 | Simple cluster, order 5 | ChiCluster descriptor | 0.52 |
| PaDEL | 2D | SC_6 | Simple cluster, order 6 | ChiCluster descriptor | 0 |
| PaDEL | 2D | VC_3 | Valence cluster, order 3 | ChiCluster descriptor | 1.72 |
| PaDEL | 2D | VC_4 | Valence cluster, order 4 | ChiCluster descriptor | 0.12 |
| PaDEL | 2D | VC_5 | Valence cluster, order 5 | ChiCluster descriptor | 0.14 |
| PaDEL | 2D | VC_6 | Valence cluster, order 6 | ChiCluster descriptor | 0 |
| PaDEL | 2D | SPC_4 | Simple path cluster, order 4 | ChiPathCluster descriptor | 5.84 |
| PaDEL | 2D | SPC_5 | Simple path cluster, order 5 | ChiPathCluster descriptor | 9.56 |
| PaDEL | 2D | SPC_6 | Simple path cluster, order 6 | ChiPathCluster descriptor | 14.92 |
| PaDEL | 2D | VPC_4 | Valence path cluster, order 4 | ChiPathCluster descriptor | 2.3 |
| PaDEL | 2D | VPC_5 | Valence path cluster, order 5 | ChiPathCluster descriptor | 2.96 |
| PaDEL | 2D | VPC_6 | Valence path cluster, order 6 | ChiPathCluster descriptor | 3.93 |
| PaDEL | 2D | SP_0 | Simple path, order 0 | PaDEL ChiPath descriptor | 21.63 |
| PaDEL | 2D | SP_1 | Simple path, order 1 | PaDEL ChiPath descriptor | 14.16 |
| PaDEL | 2D | SP_2 | Simple path, order 2 | PaDEL ChiPath descriptor | 14.2 |
| PaDEL | 2D | SP_3 | Simple path, order 3 | PaDEL ChiPath descriptor | 11.08 |
| PaDEL | 2D | SP_4 | Simple path, order 4 | PaDEL ChiPath descriptor | 9.51 |
| PaDEL | 2D | SP_5 | Simple path, order 5 | PaDEL ChiPath descriptor | 8.41 |
| PaDEL | 2D | SP_6 | Simple path, order 6 | PaDEL ChiPath descriptor | 6.81 |
| PaDEL | 2D | SP_7 | Simple path, order 7 | PaDEL ChiPath descriptor | 4.68 |
| PaDEL | 2D | ASP_0 | Average simple path, order 0 | PaDEL ChiPath descriptor | 0.72 |
| PaDEL | 2D | ASP_1 | Average simple path, order 1 | PaDEL ChiPath descriptor | 0.43 |
| PaDEL | 2D | ASP_2 | Average simple path, order 2 | PaDEL ChiPath descriptor | 0.28 |
| PaDEL | 2D | ASP_3 | Average simple path, order 3 | PaDEL ChiPath descriptor | 0.17 |
| PaDEL | 2D | ASP_4 | Average simple path, order 4 | PaDEL ChiPath descriptor | 0.1 |
| PaDEL | 2D | ASP_5 | Average simple path, order 5 | PaDEL ChiPath descriptor | 0.06 |
| PaDEL | 2D | ASP_6 | Average simple path, order 6 | PaDEL ChiPath descriptor | 0.04 |
| PaDEL | 2D | ASP_7 | Average simple path, order 7 | PaDEL ChiPath descriptor | 0.02 |
| PaDEL | 2D | VP_0 | Valence path, order 0 | PaDEL ChiPath descriptor | 17.65 |
| PaDEL | 2D | VP_1 | Valence path, order 1 | PaDEL ChiPath descriptor | 10.08 |
| PaDEL | 2D | VP_2 | Valence path, order 2 | PaDEL ChiPath descriptor | 8.62 |
| PaDEL | 2D | VP_3 | Valence path, order 3 | PaDEL ChiPath descriptor | 5.42 |
| PaDEL | 2D | VP_4 | Valence path, order 4 | PaDEL ChiPath descriptor | 3.82 |
| PaDEL | 2D | VP_5 | Valence path, order 5 | PaDEL ChiPath descriptor | 2.97 |
| PaDEL | 2D | VP_6 | Valence path, order 6 | PaDEL ChiPath descriptor | 1.94 |
| PaDEL | 2D | VP_7 | Valence path, order 7 | PaDEL ChiPath descriptor | 1.22 |
| PaDEL | 2D | AVP_0 | Average valence path, order 0 | PaDEL ChiPath descriptor | 0.59 |
| PaDEL | 2D | AVP_1 | Average valence path, order 1 | PaDEL ChiPath descriptor | 0.31 |
| PaDEL | 2D | AVP_2 | Average valence path, order 2 | PaDEL ChiPath descriptor | 0.17 |
| PaDEL | 2D | AVP_3 | Average valence path, order 3 | PaDEL ChiPath descriptor | 0.08 |
| PaDEL | 2D | AVP_4 | Average valence path, order 4 | PaDEL ChiPath descriptor | 0.04 |
| PaDEL | 2D | AVP_5 | Average valence path, order 5 | PaDEL ChiPath descriptor | 0.02 |
| PaDEL | 2D | AVP_6 | Average valence path, order 6 | PaDEL ChiPath descriptor | 0.01 |
| PaDEL | 2D | AVP_7 | Average valence path, order 7 | PaDEL ChiPath descriptor | 0.01 |
| PaDEL | 2D | Sv | Sum of atomic van der Waals volumes (scaled on carbon atom) | Constitutional descriptor | 35.62 |
| PaDEL | 2D | Sse | Sum of atomic Sanderson electronegativities (scaled on carbon atom) | Constitutional descriptor | 56.2 |
| PaDEL | 2D | Spe | Sum of atomic Pauling electronegativities (scaled on carbon atom) | Constitutional descriptor | 54.18 |
| PaDEL | 2D | Sare | Sum of atomic Allred-Rochow electronegativities (scaled on carbon atom) | Constitutional descriptor | 54.88 |
| PaDEL | 2D | Sp | Sum of atomic polarizabilities (scaled on carbon atom) | Constitutional descriptor | 37.78 |
| PaDEL | 2D | Si | Sum of first first ionization potentials (scaled on carbon atom) | Constitutional descriptor | 421.05 |
| PaDEL | 2D | Mv | Mean atomic van der Waals volumes (scaled on carbon atom) | Constitutional descriptor | 0.64 |
| PaDEL | 2D | Mse | Mean atomic Sanderson electronegativities (scaled on carbon atom) | Constitutional descriptor | 1 |
| PaDEL | 2D | Mpe | Mean atomic Pauling electronegativities (scaled on carbon atom) | Constitutional descriptor | 0.97 |
| PaDEL | 2D | Mare | Mean atomic Allred-Rochow electronegativities (scaled on carbon atom) | Constitutional descriptor | 0.98 |
| PaDEL | 2D | Mp | Mean atomic polarizabilities (scaled on carbon atom) | Constitutional descriptor | 0.67 |
| PaDEL | 2D | Mi | Mean first first ionization potentials (scaled on carbon atom) | Constitutional descriptor | 7.52 |
| PaDEL | 2D | CrippenLogP | Crippen's LogP | Crippen descriptor | 5.55 |
| PaDEL | 2D | CrippenMR | Crippen's molar refractivity | Crippen descriptor | 119.44 |
| PaDEL | 2D | SpMax_Dt | Leading eigenvalue from detour matrix | Detour Matrix descriptor | 378.69 |
| PaDEL | 2D | SpDiam_Dt | Spectral diameter from detour matrix | Detour Matrix descriptor | 487.21 |
| PaDEL | 2D | SpAD_Dt | Spectral absolute deviation from detour matrix | Detour Matrix descriptor | 759.78 |
| PaDEL | 2D | SpMAD_Dt | Spectral mean absolute deviation from detour matrix | Detour Matrix descriptor | 25.33 |
| PaDEL | 2D | EE_Dt | Estrada-like index from detour matrix | Detour Matrix descriptor | 378.69 |
| PaDEL | 2D | VE1_Dt | Coefficient sum of the last eigenvector from detour matrix | Detour Matrix descriptor | 0.06 |
| PaDEL | 2D | VE2_Dt | Average coefficient sum of the last eigenvector from detour matrix | Detour Matrix descriptor | 0 |
| PaDEL | 2D | VE3_Dt | Logarithmic coefficient sum of the last eigenvector from detour matrix | Detour Matrix descriptor | -8.27 |
| PaDEL | 2D | VR1_Dt | Randic-like eigenvector-based index from detour matrix | Detour Matrix descriptor | 678.22 |
| PaDEL | 2D | VR2_Dt | Normalized Randic-like eigenvector-based index from detour matrix | Detour Matrix descriptor | 22.61 |
| PaDEL | 2D | VR3_Dt | Logarithmic Randic-like eigenvector-based index from detour matrix | Detour Matrix descriptor | 19.56 |
| PaDEL | 2D | ECCEN | A topological descriptor combining distance and adjacency information | Eccentric Connectivity Index descriptor | 665 |
| PaDEL | 2D | nHBd | Count of E-States for (strong) Hydrogen Bond donors | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | nwHBd | Count of E-States for weak Hydrogen Bond donors | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHBa | Count of E-States for (strong) Hydrogen Bond acceptors | Electrotopological State Atom Type descriptor | 5 |
| PaDEL | 2D | nwHBa | Count of E-States for weak Hydrogen Bond acceptors | Electrotopological State Atom Type descriptor | 17 |
| PaDEL | 2D | nHBint2 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHBint3 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHBint4 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 4 | Electrotopological State Atom Type descriptor | 3 |
| PaDEL | 2D | nHBint5 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHBint6 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 6 | Electrotopological State Atom Type descriptor | 1 |
| PaDEL | 2D | nHBint7 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 7 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHBint8 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 8 | Electrotopological State Atom Type descriptor | 1 |
| PaDEL | 2D | nHBint9 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 9 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHBint10 | Count of E-State descriptors of strength for potential Hydrogen Bonds of path length 10 | Electrotopological State Atom Type descriptor | 3 |
| PaDEL | 2D | nHsOH | Count of atom-type H E-State: -OH | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | nHdNH | Count of atom-type H E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHsSH | Count of atom-type H E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHsNH2 | Count of atom-type H E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHssNH | Count of atom-type H E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHaaNH | Count of atom-type H E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHsNH3p | Count of atom-type H E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHssNH2p | Count of atom-type H E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHsssNHp | Count of atom-type H E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHtCH | Count of atom-type H E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHdCH2 | Count of atom-type H E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHdsCH | Count of atom-type H E-State: =CH- | Electrotopological State Atom Type descriptor | 3 |
| PaDEL | 2D | nHaaCH | Count of atom-type H E-State: :CH: | Electrotopological State Atom Type descriptor | 4 |
| PaDEL | 2D | nHCHnX | Count of atom-type H E-State: CHnX | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nHCsats | Count of atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 4 |
| PaDEL | 2D | nHCsatu | Count of atom-type H E-State: H on C sp3 bonded to unsaturated C | Electrotopological State Atom Type descriptor | 4 |
| PaDEL | 2D | nHAvin | Count of atom-type H E-State: H on C vinyl bonded to C aromatic | Electrotopological State Atom Type descriptor | 1 |
| PaDEL | 2D | nHother | Count of atom-type H E-State: H on aaCH, dCH2 or dsCH | Electrotopological State Atom Type descriptor | 7 |
| PaDEL | 2D | nHmisc | Count of atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsLi | Count of atom-type E-State: -Li | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssBe | Count of atom-type E-State: -Be- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssBem | Count of atom-type E-State: >Be<-2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsBH2 | Count of atom-type E-State: -BH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssBH | Count of atom-type E-State: -BH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssB | Count of atom-type E-State: -B< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssBm | Count of atom-type E-State: >B<- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsCH3 | Count of atom-type E-State: -CH3 | Electrotopological State Atom Type descriptor | 4 |
| PaDEL | 2D | ndCH2 | Count of atom-type E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssCH2 | Count of atom-type E-State: -CH2- | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | ntCH | Count of atom-type E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndsCH | Count of atom-type E-State: =CH- | Electrotopological State Atom Type descriptor | 3 |
| PaDEL | 2D | naaCH | Count of atom-type E-State: :CH: | Electrotopological State Atom Type descriptor | 4 |
| PaDEL | 2D | nsssCH | Count of atom-type E-State: >CH- | Electrotopological State Atom Type descriptor | 1 |
| PaDEL | 2D | nddC | Count of atom-type E-State: =C= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ntsC | Count of atom-type E-State: #C- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndssC | Count of atom-type E-State: =C< | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | naasC | Count of atom-type E-State: :C:- | Electrotopological State Atom Type descriptor | 8 |
| PaDEL | 2D | naaaC | Count of atom-type E-State: ::C: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssC | Count of atom-type E-State: >C< | Electrotopological State Atom Type descriptor | 1 |
| PaDEL | 2D | nsNH3p | Count of atom-type E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsNH2 | Count of atom-type E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssNH2p | Count of atom-type E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndNH | Count of atom-type E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssNH | Count of atom-type E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | naaNH | Count of atom-type E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ntN | Count of atom-type E-State: #N | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssNHp | Count of atom-type E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndsN | Count of atom-type E-State: =N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | naaN | Count of atom-type E-State: :N: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssN | Count of atom-type E-State: >N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nddsN | Count of atom-type E-State: -N<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | naasN | Count of atom-type E-State: :N:- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssNp | Count of atom-type E-State: >N<+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsOH | Count of atom-type E-State: -OH | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | ndO | Count of atom-type E-State: =O | Electrotopological State Atom Type descriptor | 1 |
| PaDEL | 2D | nssO | Count of atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | naaO | Count of atom-type E-State: :O: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | naOm | Count of atom-type E-State: :O-0.5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsOm | Count of atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsF | Count of atom-type E-State: -F | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsSiH3 | Count of atom-type E-State: -SiH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssSiH2 | Count of atom-type E-State: -SiH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssSiH | Count of atom-type E-State: >SiH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssSi | Count of atom-type E-State: >Si< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsPH2 | Count of atom-type E-State: -PH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssPH | Count of atom-type E-State: -PH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssP | Count of atom-type E-State: >P- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndsssP | Count of atom-type E-State: ->P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nddsP | Count of atom-type E-State: -=P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssssP | Count of atom-type E-State: ->P< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsSH | Count of atom-type E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndS | Count of atom-type E-State: =S | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssS | Count of atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | naaS | Count of atom-type E-State: aSa | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndssS | Count of atom-type E-State: >S= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nddssS | Count of atom-type E-State: >S== | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssssS | Count of atom-type E-State: >S<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nSm | Count of atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsCl | Count of atom-type E-State: -Cl | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsGeH3 | Count of atom-type E-State: -GeH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssGeH2 | Count of atom-type E-State: -GeH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssGeH | Count of atom-type E-State: >GeH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssGe | Count of atom-type E-State: >Ge< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsAsH2 | Count of atom-type E-State: -AsH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssAsH | Count of atom-type E-State: -AsH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssAs | Count of atom-type E-State: >As- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndsssAs | Count of atom-type E-State: ->As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nddsAs | Count of atom-type E-State: -=As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssssAs | Count of atom-type E-State: ->As< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsSeH | Count of atom-type E-State: -SeH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndSe | Count of atom-type E-State: =Se | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssSe | Count of atom-type E-State: -Se- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | naaSe | Count of atom-type E-State: aSea | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | ndssSe | Count of atom-type E-State: >Se= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssssSe | Count of atom-type E-State: >Se<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nddssSe | Count of atom-type E-State: -=Se=- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsBr | Count of atom-type E-State: -Br | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsSnH3 | Count of atom-type E-State: -SnH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssSnH2 | Count of atom-type E-State: -SnH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssSnH | Count of atom-type E-State: >SnH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssSn | Count of atom-type E-State: >Sn< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsI | Count of atom-type E-State: -I | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsPbH3 | Count of atom-type E-State: -PbH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssPbH2 | Count of atom-type E-State: -PbH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nsssPbH | Count of atom-type E-State: >PbH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | nssssPb | Count of atom-type E-State: >Pb< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBd | Sum of E-States for (strong) hydrogen bond donors | Electrotopological State Atom Type descriptor | 0.85 |
| PaDEL | 2D | SwHBd | Sum of E-States for weak hydrogen bond donors | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBa | Sum of E-States for (strong) hydrogen bond acceptors | Electrotopological State Atom Type descriptor | 46.19 |
| PaDEL | 2D | SwHBa | Sum of E-States for weak hydrogen bond acceptors | Electrotopological State Atom Type descriptor | 16.66 |
| PaDEL | 2D | SHBint2 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBint3 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBint4 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 4 | Electrotopological State Atom Type descriptor | 7.51 |
| PaDEL | 2D | SHBint5 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBint6 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 6 | Electrotopological State Atom Type descriptor | 3.48 |
| PaDEL | 2D | SHBint7 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 7 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBint8 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 8 | Electrotopological State Atom Type descriptor | 7.29 |
| PaDEL | 2D | SHBint9 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 9 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHBint10 | Sum of E-State descriptors of strength for potential hydrogen bonds of path length 10 | Electrotopological State Atom Type descriptor | 12.44 |
| PaDEL | 2D | SHsOH | Sum of atom-type H E-State: -OH | Electrotopological State Atom Type descriptor | 0.85 |
| PaDEL | 2D | SHdNH | Sum of atom-type H E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHsSH | Sum of atom-type H E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHsNH2 | Sum of atom-type H E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHssNH | Sum of atom-type H E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHaaNH | Sum of atom-type H E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHsNH3p | Sum of atom-type H E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHssNH2p | Sum of atom-type H E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHsssNHp | Sum of atom-type H E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHtCH | Sum of atom-type H E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHdCH2 | Sum of atom-type H E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHdsCH | Sum of atom-type H E-State: =CH- | Electrotopological State Atom Type descriptor | 1.37 |
| PaDEL | 2D | SHaaCH | Sum of atom-type H E-State: :CH: | Electrotopological State Atom Type descriptor | 2.2 |
| PaDEL | 2D | SHCHnX | Sum of atom-type H E-State: CHnX | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SHCsats | Sum of atom-type H E-State: H on C sp3 bonded to saturated C | Electrotopological State Atom Type descriptor | 2.69 |
| PaDEL | 2D | SHCsatu | Sum of atom-type H E-State: H on C sp3 bonded to unsaturated C | Electrotopological State Atom Type descriptor | 2.32 |
| PaDEL | 2D | SHAvin | Sum of atom-type H E-State: H on C vinyl bonded to C aromatic | Electrotopological State Atom Type descriptor | 0.51 |
| PaDEL | 2D | SHother | Sum of atom-type H E-State: H on aaCH, dCH2 or dsCH | Electrotopological State Atom Type descriptor | 3.58 |
| PaDEL | 2D | SHmisc | Sum of atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsLi | Sum of atom-type E-State: -Li | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssBe | Sum of atom-type E-State: -Be- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssBem | Sum of atom-type E-State: >Be<-2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsBH2 | Sum of atom-type E-State: -BH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssBH | Sum of atom-type E-State: -BH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssB | Sum of atom-type E-State: -B< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssBm | Sum of atom-type E-State: >B<- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsCH3 | Sum of atom-type E-State: -CH3 | Electrotopological State Atom Type descriptor | 7.87 |
| PaDEL | 2D | SdCH2 | Sum of atom-type E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssCH2 | Sum of atom-type E-State: -CH2- | Electrotopological State Atom Type descriptor | 0.57 |
| PaDEL | 2D | StCH | Sum of atom-type E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdsCH | Sum of atom-type E-State: =CH- | Electrotopological State Atom Type descriptor | 5.84 |
| PaDEL | 2D | SaaCH | Sum of atom-type E-State: :CH: | Electrotopological State Atom Type descriptor | 6.62 |
| PaDEL | 2D | SsssCH | Sum of atom-type E-State: >CH- | Electrotopological State Atom Type descriptor | -0.5 |
| PaDEL | 2D | SddC | Sum of atom-type E-State: =C= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | StsC | Sum of atom-type E-State: #C- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdssC | Sum of atom-type E-State: =C< | Electrotopological State Atom Type descriptor | 0.94 |
| PaDEL | 2D | SaasC | Sum of atom-type E-State: :C:- | Electrotopological State Atom Type descriptor | 3.27 |
| PaDEL | 2D | SaaaC | Sum of atom-type E-State: ::C: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssC | Sum of atom-type E-State: >C< | Electrotopological State Atom Type descriptor | -0.54 |
| PaDEL | 2D | SsNH3p | Sum of atom-type E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsNH2 | Sum of atom-type E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssNH2p | Sum of atom-type E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdNH | Sum of atom-type E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssNH | Sum of atom-type E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SaaNH | Sum of atom-type E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | StN | Sum of atom-type E-State: #N | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssNHp | Sum of atom-type E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdsN | Sum of atom-type E-State: =N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SaaN | Sum of atom-type E-State: :N: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssN | Sum of atom-type E-State: >N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SddsN | Sum of atom-type E-State: -N<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SaasN | Sum of atom-type E-State: :N:- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssNp | Sum of atom-type E-State: >N<+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsOH | Sum of atom-type E-State: -OH | Electrotopological State Atom Type descriptor | 20.63 |
| PaDEL | 2D | SdO | Sum of atom-type E-State: =O | Electrotopological State Atom Type descriptor | 13.09 |
| PaDEL | 2D | SssO | Sum of atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 12.47 |
| PaDEL | 2D | SaaO | Sum of atom-type E-State: :O: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SaOm | Sum of atom-type E-State: :O-0.5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsOm | Sum of atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsF | Sum of atom-type E-State: -F | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsSiH3 | Sum of atom-type E-State: -SiH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssSiH2 | Sum of atom-type E-State: -SiH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssSiH | Sum of atom-type E-State: >SiH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssSi | Sum of atom-type E-State: >Si< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsPH2 | Sum of atom-type E-State: -PH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssPH | Sum of atom-type E-State: -PH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssP | Sum of atom-type E-State: >P- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdsssP | Sum of atom-type E-State: ->P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SddsP | Sum of atom-type E-State: -=P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssssP | Sum of atom-type E-State: ->P< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsSH | Sum of atom-type E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdS | Sum of atom-type E-State: =S | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssS | Sum of atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SaaS | Sum of atom-type E-State: aSa | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdssS | Sum of atom-type E-State: >S= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SddssS | Sum of atom-type E-State: >S== | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssssS | Sum of atom-type E-State: >S<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SSm | Sum of atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsCl | Sum of atom-type E-State: -Cl | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsGeH3 | Sum of atom-type E-State: -GeH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssGeH2 | Sum of atom-type E-State: -GeH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssGeH | Sum of atom-type E-State: >GeH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssGe | Sum of atom-type E-State: >Ge< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsAsH2 | Sum of atom-type E-State: -AsH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssAsH | Sum of atom-type E-State: -AsH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssAs | Sum of atom-type E-State: >As- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdsssAs | Sum of atom-type E-State: ->As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SddsAs | Sum of atom-type E-State: -=As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssssAs | Sum of atom-type E-State: ->As< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsSeH | Sum of atom-type E-State: -SeH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdSe | Sum of atom-type E-State: =Se | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssSe | Sum of atom-type E-State: -Se- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SaaSe | Sum of atom-type E-State: aSea | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SdssSe | Sum of atom-type E-State: >Se= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssssSe | Sum of atom-type E-State: >Se<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SddssSe | Sum of atom-type E-State: -=Se=- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsBr | Sum of atom-type E-State: -Br | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsSnH3 | Sum of atom-type E-State: -SnH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssSnH2 | Sum of atom-type E-State: -SnH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssSnH | Sum of atom-type E-State: >SnH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssSn | Sum of atom-type E-State: >Sn< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsI | Sum of atom-type E-State: -I | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsPbH3 | Sum of atom-type E-State: -PbH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssPbH2 | Sum of atom-type E-State: -PbH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SsssPbH | Sum of atom-type E-State: >PbH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | SssssPb | Sum of atom-type E-State: >Pb< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBd | Minimum E-States for (strong) Hydrogen Bond donors | Electrotopological State Atom Type descriptor | 0.29 |
| PaDEL | 2D | minwHBd | Minimum E-States for weak Hydrogen Bond donors | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBa | Minimum E-States for (strong) Hydrogen Bond acceptors | Electrotopological State Atom Type descriptor | 6.22 |
| PaDEL | 2D | minwHBa | Minimum E-States for weak Hydrogen Bond acceptors | Electrotopological State Atom Type descriptor | -0.17 |
| PaDEL | 2D | minHBint2 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBint3 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBint4 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 4 | Electrotopological State Atom Type descriptor | 1.83 |
| PaDEL | 2D | minHBint5 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBint6 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 6 | Electrotopological State Atom Type descriptor | 3.48 |
| PaDEL | 2D | minHBint7 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 7 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBint8 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 8 | Electrotopological State Atom Type descriptor | 7.29 |
| PaDEL | 2D | minHBint9 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 9 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHBint10 | Minimum E-State descriptors of strength for potential Hydrogen Bonds of path length 10 | Electrotopological State Atom Type descriptor | 2.81 |
| PaDEL | 2D | minHsOH | Minimum atom-type H E-State: -OH | Electrotopological State Atom Type descriptor | 0.29 |
| PaDEL | 2D | minHdNH | Minimum atom-type H E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHsSH | Minimum atom-type H E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHsNH2 | Minimum atom-type H E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHssNH | Minimum atom-type H E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHaaNH | Minimum atom-type H E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHsNH3p | Minimum atom-type H E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHssNH2p | Minimum atom-type H E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHsssNHp | Minimum atom-type H E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHtCH | Minimum atom-type H E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHdCH2 | Minimum atom-type H E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHdsCH | Minimum atom-type H E-State: =CH- | Electrotopological State Atom Type descriptor | 0.41 |
| PaDEL | 2D | minHaaCH | Minimum atom-type H E-State: :CH: | Electrotopological State Atom Type descriptor | 0.54 |
| PaDEL | 2D | minHCHnX | Minimum atom-type H E-State: CHnX | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minHCsats | Minimum atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 0.5 |
| PaDEL | 2D | minHCsatu | Minimum atom-type H E-State: H on C sp3 bonded to unsaturated C | Electrotopological State Atom Type descriptor | 0.42 |
| PaDEL | 2D | minHAvin | Minimum atom-type H E-State: H on C vinyl bonded to C aromatic | Electrotopological State Atom Type descriptor | 0.51 |
| PaDEL | 2D | minHother | Minimum atom-type H E-State: H on aaCH, dCH2 or dsCH | Electrotopological State Atom Type descriptor | 0.41 |
| PaDEL | 2D | minHmisc | Minimum atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsLi | Minimum atom-type E-State: -Li | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssBe | Minimum atom-type E-State: -Be- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssBem | Minimum atom-type E-State: >Be<-2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsBH2 | Minimum atom-type E-State: -BH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssBH | Minimum atom-type E-State: -BH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssB | Minimum atom-type E-State: -B< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssBm | Minimum atom-type E-State: >B<- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsCH3 | Minimum atom-type E-State: -CH3 | Electrotopological State Atom Type descriptor | 1.94 |
| PaDEL | 2D | mindCH2 | Minimum atom-type E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssCH2 | Minimum atom-type E-State: -CH2- | Electrotopological State Atom Type descriptor | 0.11 |
| PaDEL | 2D | mintCH | Minimum atom-type E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindsCH | Minimum atom-type E-State: =CH- | Electrotopological State Atom Type descriptor | 1.91 |
| PaDEL | 2D | minaaCH | Minimum atom-type E-State: :CH: | Electrotopological State Atom Type descriptor | 1.57 |
| PaDEL | 2D | minsssCH | Minimum atom-type E-State: >CH- | Electrotopological State Atom Type descriptor | -0.5 |
| PaDEL | 2D | minddC | Minimum atom-type E-State: =C= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mintsC | Minimum atom-type E-State: #C- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindssC | Minimum atom-type E-State: =C< | Electrotopological State Atom Type descriptor | -0.17 |
| PaDEL | 2D | minaasC | Minimum atom-type E-State: :C:- | Electrotopological State Atom Type descriptor | -0.07 |
| PaDEL | 2D | minaaaC | Minimum atom-type E-State: ::C: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssC | Minimum atom-type E-State: >C< | Electrotopological State Atom Type descriptor | -0.54 |
| PaDEL | 2D | minsNH3p | Minimum atom-type E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsNH2 | Minimum atom-type E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssNH2p | Minimum atom-type E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindNH | Minimum atom-type E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssNH | Minimum atom-type E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minaaNH | Minimum atom-type E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mintN | Minimum atom-type E-State: #N | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssNHp | Minimum atom-type E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindsN | Minimum atom-type E-State: =N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minaaN | Minimum atom-type E-State: :N: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssN | Minimum atom-type E-State: >N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minddsN | Minimum atom-type E-State: -N<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minaasN | Minimum atom-type E-State: :N:- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssNp | Minimum atom-type E-State: >N<+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsOH | Minimum atom-type E-State: -OH | Electrotopological State Atom Type descriptor | 9.56 |
| PaDEL | 2D | mindO | Minimum atom-type E-State: =O | Electrotopological State Atom Type descriptor | 13.09 |
| PaDEL | 2D | minssO | Minimum atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 6.22 |
| PaDEL | 2D | minaaO | Minimum atom-type E-State: :O: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minaOm | Minimum atom-type E-State: :O-0.5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsOm | Minimum atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsF | Minimum atom-type E-State: -F | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsSiH3 | Minimum atom-type E-State: -SiH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssSiH2 | Minimum atom-type E-State: -SiH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssSiH | Minimum atom-type E-State: >SiH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssSi | Minimum atom-type E-State: >Si< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsPH2 | Minimum atom-type E-State: -PH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssPH | Minimum atom-type E-State: -PH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssP | Minimum atom-type E-State: >P- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindsssP | Minimum atom-type E-State: ->P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minddsP | Minimum atom-type E-State: -=P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssssP | Minimum atom-type E-State: ->P< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsSH | Minimum atom-type E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindS | Minimum atom-type E-State: =S | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssS | Minimum atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minaaS | Minimum atom-type E-State: aSa | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindssS | Minimum atom-type E-State: >S= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minddssS | Minimum atom-type E-State: >S== | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssssS | Minimum atom-type E-State: >S<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minSm | Minimum atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsCl | Minimum atom-type E-State: -Cl | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsGeH3 | Minimum atom-type E-State: -GeH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssGeH2 | Minimum atom-type E-State: -GeH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssGeH | Minimum atom-type E-State: >GeH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssGe | Minimum atom-type E-State: >Ge< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsAsH2 | Minimum atom-type E-State: -AsH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssAsH | Minimum atom-type E-State: -AsH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssAs | Minimum atom-type E-State: >As- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindsssAs | Minimum atom-type E-State: ->As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minddsAs | Minimum atom-type E-State: -=As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssssAs | Minimum atom-type E-State: ->As< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsSeH | Minimum atom-type E-State: -SeH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindSe | Minimum atom-type E-State: =Se | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssSe | Minimum atom-type E-State: -Se- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minaaSe | Minimum atom-type E-State: aSea | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | mindssSe | Minimum atom-type E-State: >Se= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssssSe | Minimum atom-type E-State: >Se<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minddssSe | Minimum atom-type E-State: -=Se=- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsBr | Minimum atom-type E-State: -Br | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsSnH3 | Minimum atom-type E-State: -SnH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssSnH2 | Minimum atom-type E-State: -SnH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssSnH | Minimum atom-type E-State: >SnH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssSn | Minimum atom-type E-State: >Sn< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsI | Minimum atom-type E-State: -I | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsPbH3 | Minimum atom-type E-State: -PbH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssPbH2 | Minimum atom-type E-State: -PbH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minsssPbH | Minimum atom-type E-State: >PbH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | minssssPb | Minimum atom-type E-State: >Pb< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBd | Maximum E-States for (strong) Hydrogen Bond donors | Electrotopological State Atom Type descriptor | 0.56 |
| PaDEL | 2D | maxwHBd | Maximum E-States for weak Hydrogen Bond donors | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBa | Maximum E-States for (strong) Hydrogen Bond acceptors | Electrotopological State Atom Type descriptor | 13.09 |
| PaDEL | 2D | maxwHBa | Maximum E-States for weak Hydrogen Bond acceptors | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | maxHBint2 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBint3 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBint4 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 4 | Electrotopological State Atom Type descriptor | 3.85 |
| PaDEL | 2D | maxHBint5 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBint6 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 6 | Electrotopological State Atom Type descriptor | 3.48 |
| PaDEL | 2D | maxHBint7 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 7 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBint8 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 8 | Electrotopological State Atom Type descriptor | 7.29 |
| PaDEL | 2D | maxHBint9 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 9 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHBint10 | Maximum E-State descriptors of strength for potential Hydrogen Bonds of path length 10 | Electrotopological State Atom Type descriptor | 6.17 |
| PaDEL | 2D | maxHsOH | Maximum atom-type H E-State: -OH | Electrotopological State Atom Type descriptor | 0.56 |
| PaDEL | 2D | maxHdNH | Maximum atom-type H E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHsSH | Maximum atom-type H E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHsNH2 | Maximum atom-type H E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHssNH | Maximum atom-type H E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHaaNH | Maximum atom-type H E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHsNH3p | Maximum atom-type H E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHssNH2p | Maximum atom-type H E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHsssNHp | Maximum atom-type H E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHtCH | Maximum atom-type H E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHdCH2 | Maximum atom-type H E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHdsCH | Maximum atom-type H E-State: =CH- | Electrotopological State Atom Type descriptor | 0.51 |
| PaDEL | 2D | maxHaaCH | Maximum atom-type H E-State: :CH: | Electrotopological State Atom Type descriptor | 0.56 |
| PaDEL | 2D | maxHCHnX | Maximum atom-type H E-State: CHnX | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxHCsats | Maximum atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 0.91 |
| PaDEL | 2D | maxHCsatu | Maximum atom-type H E-State: H on C sp3 bonded to unsaturated C | Electrotopological State Atom Type descriptor | 0.79 |
| PaDEL | 2D | maxHAvin | Maximum atom-type H E-State: H on C vinyl bonded to C aromatic | Electrotopological State Atom Type descriptor | 0.51 |
| PaDEL | 2D | maxHother | Maximum atom-type H E-State: H on aaCH, dCH2 or dsCH | Electrotopological State Atom Type descriptor | 0.56 |
| PaDEL | 2D | maxHmisc | Maximum atom-type H E-State: H bonded to B, Si, P, Ge, As, Se, Sn or Pb | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsLi | Maximum atom-type E-State: -Li | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssBe | Maximum atom-type E-State: -Be- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssBem | Maximum atom-type E-State: >Be<-2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsBH2 | Maximum atom-type E-State: -BH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssBH | Maximum atom-type E-State: -BH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssB | Maximum atom-type E-State: -B< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssBm | Maximum atom-type E-State: >B<- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsCH3 | Maximum atom-type E-State: -CH3 | Electrotopological State Atom Type descriptor | 1.99 |
| PaDEL | 2D | maxdCH2 | Maximum atom-type E-State: =CH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssCH2 | Maximum atom-type E-State: -CH2- | Electrotopological State Atom Type descriptor | 0.46 |
| PaDEL | 2D | maxtCH | Maximum atom-type E-State: #CH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdsCH | Maximum atom-type E-State: =CH- | Electrotopological State Atom Type descriptor | 2 |
| PaDEL | 2D | maxaaCH | Maximum atom-type E-State: :CH: | Electrotopological State Atom Type descriptor | 1.74 |
| PaDEL | 2D | maxsssCH | Maximum atom-type E-State: >CH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxddC | Maximum atom-type E-State: =C= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxtsC | Maximum atom-type E-State: #C- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdssC | Maximum atom-type E-State: =C< | Electrotopological State Atom Type descriptor | 1.11 |
| PaDEL | 2D | maxaasC | Maximum atom-type E-State: :C:- | Electrotopological State Atom Type descriptor | 0.79 |
| PaDEL | 2D | maxaaaC | Maximum atom-type E-State: ::C: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssC | Maximum atom-type E-State: >C< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsNH3p | Maximum atom-type E-State: -NH3+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsNH2 | Maximum atom-type E-State: -NH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssNH2p | Maximum atom-type E-State: -NH2-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdNH | Maximum atom-type E-State: =NH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssNH | Maximum atom-type E-State: -NH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxaaNH | Maximum atom-type E-State: :NH: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxtN | Maximum atom-type E-State: #N | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssNHp | Maximum atom-type E-State: >NH-+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdsN | Maximum atom-type E-State: =N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxaaN | Maximum atom-type E-State: :N: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssN | Maximum atom-type E-State: >N- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxddsN | Maximum atom-type E-State: -N<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxaasN | Maximum atom-type E-State: :N:- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssNp | Maximum atom-type E-State: >N<+ | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsOH | Maximum atom-type E-State: -OH | Electrotopological State Atom Type descriptor | 11.07 |
| PaDEL | 2D | maxdO | Maximum atom-type E-State: =O | Electrotopological State Atom Type descriptor | 13.09 |
| PaDEL | 2D | maxssO | Maximum atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 6.25 |
| PaDEL | 2D | maxaaO | Maximum atom-type E-State: :O: | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxaOm | Maximum atom-type E-State: :O-0.5 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsOm | Maximum atom-type E-State: -O- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsF | Maximum atom-type E-State: -F | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsSiH3 | Maximum atom-type E-State: -SiH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssSiH2 | Maximum atom-type E-State: -SiH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssSiH | Maximum atom-type E-State: >SiH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssSi | Maximum atom-type E-State: >Si< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsPH2 | Maximum atom-type E-State: -PH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssPH | Maximum atom-type E-State: -PH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssP | Maximum atom-type E-State: >P- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdsssP | Maximum atom-type E-State: ->P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxddsP | Maximum atom-type E-State: -=P= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssssP | Maximum atom-type E-State: ->P< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsSH | Maximum atom-type E-State: -SH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdS | Maximum atom-type E-State: =S | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssS | Maximum atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxaaS | Maximum atom-type E-State: aSa | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdssS | Maximum atom-type E-State: >S= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxddssS | Maximum atom-type E-State: >S== | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssssS | Maximum atom-type E-State: >S<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxSm | Maximum atom-type E-State: -S- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsCl | Maximum atom-type E-State: -Cl | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsGeH3 | Maximum atom-type E-State: -GeH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssGeH2 | Maximum atom-type E-State: -GeH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssGeH | Maximum atom-type E-State: >GeH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssGe | Maximum atom-type E-State: >Ge< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsAsH2 | Maximum atom-type E-State: -AsH2 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssAsH | Maximum atom-type E-State: -AsH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssAs | Maximum atom-type E-State: >As- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdsssAs | Maximum atom-type E-State: ->As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxddsAs | Maximum atom-type E-State: -=As= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssssAs | Maximum atom-type E-State: ->As< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsSeH | Maximum atom-type E-State: -SeH | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdSe | Maximum atom-type E-State: =Se | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssSe | Maximum atom-type E-State: -Se- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxaaSe | Maximum atom-type E-State: aSea | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxdssSe | Maximum atom-type E-State: >Se= | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssssSe | Maximum atom-type E-State: >Se<< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxddssSe | Maximum atom-type E-State: -=Se=- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsBr | Maximum atom-type E-State: -Br | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsSnH3 | Maximum atom-type E-State: -SnH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssSnH2 | Maximum atom-type E-State: -SnH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssSnH | Maximum atom-type E-State: >SnH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssSn | Maximum atom-type E-State: >Sn< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsI | Maximum atom-type E-State: -I | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsPbH3 | Maximum atom-type E-State: -PbH3 | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssPbH2 | Maximum atom-type E-State: -PbH2- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxsssPbH | Maximum atom-type E-State: >PbH- | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | maxssssPb | Maximum atom-type E-State: >Pb< | Electrotopological State Atom Type descriptor | 0 |
| PaDEL | 2D | sumI | Sum of the intrinsic state values I | Electrotopological State Atom Type descriptor | 70.25 |
| PaDEL | 2D | meanI | Mean intrinsic state values I | Electrotopological State Atom Type descriptor | 2.34 |
| PaDEL | 2D | hmax | Maximum H E-State | Electrotopological State Atom Type descriptor | 0.91 |
| PaDEL | 2D | gmax | Maximum E-State | Electrotopological State Atom Type descriptor | 13.09 |
| PaDEL | 2D | hmin | Minimum H E-State | Electrotopological State Atom Type descriptor | -0.36 |
| PaDEL | 2D | gmin | Minimum E-State | Electrotopological State Atom Type descriptor | -0.54 |
| PaDEL | 2D | LipoaffinityIndex | Lipoaffinity index | Electrotopological State Atom Type descriptor | 7.47 |
| PaDEL | 2D | MAXDN | Maximum negative intrinsic state difference in the molecule (related to the nucleophilicity of the | Electrotopological State Atom Type descriptor | 1.84 |
| PaDEL | 2D | MAXDP | Maximum positive intrinsic state difference in the molecule (related to the electrophilicity of the | Electrotopological State Atom Type descriptor | 6.09 |
| PaDEL | 2D | DELS | Sum of all atoms intrinsic state differences (measure of total charge transfer in the molecule). Us | Electrotopological State Atom Type descriptor | 40.38 |
| PaDEL | 2D | MAXDN2 | Maximum negative intrinsic state difference in the molecule (related to the nucleophilicity of the | Electrotopological State Atom Type descriptor | 1.84 |
| PaDEL | 2D | MAXDP2 | Maximum positive intrinsic state difference in the molecule (related to the electrophilicity of the | Electrotopological State Atom Type descriptor | 6.09 |
| PaDEL | 2D | DELS2 | Sum of all atoms intrinsic state differences (measure of total charge transfer in the molecule). Usi | Electrotopological State Atom Type descriptor | 40.38 |
| PaDEL | 2D | ETA_Alpha | Sum of alpha values of all non-hydrogen vertices of a molecule | Extended Topochemical Atom descriptor | 14.17 |
| PaDEL | 2D | ETA_AlphaP | Sum of alpha values of all non-hydrogen vertices of a molecule relative to molecular size | Extended Topochemical Atom descriptor | 0.47 |
| PaDEL | 2D | ETA_dAlpha_A | A measure of count of non-hydrogen heteroatoms | Extended Topochemical Atom descriptor | 0 |
| PaDEL | 2D | ETA_dAlpha_B | A measure of count of hydrogen bond acceptor atoms and/or polar surface area | Extended Topochemical Atom descriptor | 0.03 |
| PaDEL | 2D | ETA_Epsilon_1 | A measure of electronegative atom count | Extended Topochemical Atom descriptor | 0.58 |
| PaDEL | 2D | ETA_Epsilon_2 | A measure of electronegative atom count | Extended Topochemical Atom descriptor | 0.83 |
| PaDEL | 2D | ETA_Epsilon_3 | A measure of electronegative atom count | Extended Topochemical Atom descriptor | 0.44 |
| PaDEL | 2D | ETA_Epsilon_4 | A measure of electronegative atom count | Extended Topochemical Atom descriptor | 0.52 |
| PaDEL | 2D | ETA_Epsilon_5 | A measure of electronegative atom count | Extended Topochemical Atom descriptor | 0.79 |
| PaDEL | 2D | ETA_dEpsilon_A | A measure of contribution of unsaturation and electronegative atom count | Extended Topochemical Atom descriptor | 0.14 |
| PaDEL | 2D | ETA_dEpsilon_B | A measure of contribution of unsaturation | Extended Topochemical Atom descriptor | 0.06 |
| PaDEL | 2D | ETA_dEpsilon_C | A measure of contribution of electronegativity | Extended Topochemical Atom descriptor | -0.08 |
| PaDEL | 2D | ETA_dEpsilon_D | A measure of contribution of hydrogen bond donor atoms | Extended Topochemical Atom descriptor | 0.03 |
| PaDEL | 2D | ETA_Psi_1 | A measure of hydrogen bonding propensity of the molecules and/or polar surface area | Extended Topochemical Atom descriptor | 0.57 |
| PaDEL | 2D | ETA_dPsi_A | A measure of hydrogen bonding propensity of the molecules | Extended Topochemical Atom descriptor | 0.14 |
| PaDEL | 2D | ETA_dPsi_B | A measure of hydrogen bonding propensity of the molecules | Extended Topochemical Atom descriptor | 0 |
| PaDEL | 2D | ETA_Shape_P | Shape index P | Extended Topochemical Atom descriptor | 0.21 |
| PaDEL | 2D | ETA_Shape_Y | Shape index Y | Extended Topochemical Atom descriptor | 0.39 |
| PaDEL | 2D | ETA_Shape_X | Shape index X | Extended Topochemical Atom descriptor | 0.04 |
| PaDEL | 2D | ETA_Beta | A measure of electronic features of the molecule | Extended Topochemical Atom descriptor | 35.25 |
| PaDEL | 2D | ETA_BetaP | A measure of electronic features of the molecule relative to molecular size | Extended Topochemical Atom descriptor | 1.18 |
| PaDEL | 2D | ETA_Beta_s | A measure of electronegative atom count of the molecule | Extended Topochemical Atom descriptor | 18.25 |
| PaDEL | 2D | ETA_BetaP_s | A measure of electronegative atom count of the molecule relative to molecular size | Extended Topochemical Atom descriptor | 0.61 |
| PaDEL | 2D | ETA_Beta_ns | A measure of electron-richness of the molecule | Extended Topochemical Atom descriptor | 17 |
| PaDEL | 2D | ETA_BetaP_ns | A measure of electron-richness of the molecule relative to molecular size | Extended Topochemical Atom descriptor | 0.57 |
| PaDEL | 2D | ETA_dBeta | A measure of relative unsaturation content | Extended Topochemical Atom descriptor | -1.25 |
| PaDEL | 2D | ETA_dBetaP | A measure of relative unsaturation content relative to molecular size | Extended Topochemical Atom descriptor | -0.04 |
| PaDEL | 2D | ETA_Beta_ns_d | A measure of lone electrons entering into resonance | Extended Topochemical Atom descriptor | 1 |
| PaDEL | 2D | ETA_BetaP_ns_d | A measure of lone electrons entering into resonance relative to molecular size | Extended Topochemical Atom descriptor | 0.03 |
| PaDEL | 2D | ETA_Eta | Composite index Eta | Extended Topochemical Atom descriptor | 31.27 |
| PaDEL | 2D | ETA_EtaP | Composite index Eta relative to molecular size | Extended Topochemical Atom descriptor | 1.04 |
| PaDEL | 2D | ETA_Eta_R | Composite index Eta for reference alkane | Extended Topochemical Atom descriptor | 60.6 |
| PaDEL | 2D | ETA_Eta_F | Functionality index EtaF | Extended Topochemical Atom descriptor | 29.33 |
| PaDEL | 2D | ETA_EtaP_F | Functionality index EtaF relative to molecular size | Extended Topochemical Atom descriptor | 0.98 |
| PaDEL | 2D | ETA_Eta_L | Local index Eta_local | Extended Topochemical Atom descriptor | 7.38 |
| PaDEL | 2D | ETA_EtaP_L | Local index Eta_local relative to molecular size | Extended Topochemical Atom descriptor | 0.25 |
| PaDEL | 2D | ETA_Eta_R_L | Local index Eta_local for reference alkane | Extended Topochemical Atom descriptor | 14.16 |
| PaDEL | 2D | ETA_Eta_F_L | Local functionality contribution EtaF_local | Extended Topochemical Atom descriptor | 6.78 |
| PaDEL | 2D | ETA_EtaP_F_L | Local functionality contribution EtaF_local relative to molecular size | Extended Topochemical Atom descriptor | 0.23 |
| PaDEL | 2D | ETA_Eta_B | Branching index EtaB | Extended Topochemical Atom descriptor | 0.75 |
| PaDEL | 2D | ETA_EtaP_B | Branching index EtaB relative to molecular size | Extended Topochemical Atom descriptor | 0.03 |
| PaDEL | 2D | ETA_Eta_B_RC | Branching index EtaB (with ring correction) | Extended Topochemical Atom descriptor | 1.1 |
| PaDEL | 2D | ETA_EtaP_B_RC | Branching index EtaB (with ring correction) relative to molecular size | Extended Topochemical Atom descriptor | 0.04 |
| PaDEL | 2D | FMF | Complexity of a molecule | FMF descriptor | 0.36 |
| PaDEL | 2D | fragC | Complexity of a system | PaDEL Fragment Complexity descriptor | 219.05 |
| PaDEL | 2D | nHBAcc | Number of hydrogen bond acceptors (using CDK HBondAcceptorCountDescriptor algorithm) | PaDEL HBond Acceptor Count descriptor | 1 |
| PaDEL | 2D | nHBAcc2 | Number of hydrogen bond acceptors (any oxygen; any nitrogen where the formal charge of the nitrogen | PaDEL HBond Acceptor Count descriptor | 5 |
| PaDEL | 2D | nHBAcc3 | Number of hydrogen bond acceptors (any oxygen; any nitrogen where the formal charge of the nitrogen | PaDEL HBond Acceptor Count descriptor | 5 |
| PaDEL | 2D | nHBAcc_Lipinski | Number of hydrogen bond acceptors (using Lipinski's definition: any nitrogen; any oxygen) | PaDEL HBond Acceptor Count descriptor | 5 |
| PaDEL | 2D | nHBDon | Number of hydrogen bond donors (using CDK HBondDonorCountDescriptor algorithm) | PaDEL HBond Donor Count descriptor | 2 |
| PaDEL | 2D | nHBDon_Lipinski | Number of hydrogen bond donors (using Lipinski's definition: Any OH or NH. Each available hydrogen a | PaDEL HBond Donor Count descriptor | 2 |
| PaDEL | 2D | HybRatio | Fraction of sp3 carbons to sp2 carbons | Hybridization Ratio descriptor | 0.32 |
| PaDEL | 2D | IC0 | Information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 1.34 |
| PaDEL | 2D | IC1 | Information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 3.64 |
| PaDEL | 2D | IC2 | Information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 4.79 |
| PaDEL | 2D | IC3 | Information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 4.93 |
| PaDEL | 2D | IC4 | Information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 4.97 |
| PaDEL | 2D | IC5 | Information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 4.97 |
| PaDEL | 2D | TIC0 | Total information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 75.29 |
| PaDEL | 2D | TIC1 | Total information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 204.07 |
| PaDEL | 2D | TIC2 | Total information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 268.19 |
| PaDEL | 2D | TIC3 | Total information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 276.19 |
| PaDEL | 2D | TIC4 | Total information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 278.19 |
| PaDEL | 2D | TIC5 | Total information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 278.19 |
| PaDEL | 2D | SIC0 | Structural information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 0.23 |
| PaDEL | 2D | SIC1 | Structural information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 0.63 |
| PaDEL | 2D | SIC2 | Structural information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 0.82 |
| PaDEL | 2D | SIC3 | Structural information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 0.85 |
| PaDEL | 2D | SIC4 | Structural information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 0.86 |
| PaDEL | 2D | SIC5 | Structural information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 0.86 |
| PaDEL | 2D | CIC0 | Complementary information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 4.46 |
| PaDEL | 2D | CIC1 | Complementary information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 2.16 |
| PaDEL | 2D | CIC2 | Complementary information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 1.02 |
| PaDEL | 2D | CIC3 | Complementary information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 0.88 |
| PaDEL | 2D | CIC4 | Complementary information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 0.84 |
| PaDEL | 2D | CIC5 | Complementary information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 0.84 |
| PaDEL | 2D | BIC0 | Bond information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 0.22 |
| PaDEL | 2D | BIC1 | Bond information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 0.6 |
| PaDEL | 2D | BIC2 | Bond information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 0.79 |
| PaDEL | 2D | BIC3 | Bond information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 0.81 |
| PaDEL | 2D | BIC4 | Bond information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 0.82 |
| PaDEL | 2D | BIC5 | Bond information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 0.82 |
| PaDEL | 2D | MIC0 | Modified information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 11.73 |
| PaDEL | 2D | MIC1 | Modified information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 33.77 |
| PaDEL | 2D | MIC2 | Modified information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 38.99 |
| PaDEL | 2D | MIC3 | Modified information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 39.67 |
| PaDEL | 2D | MIC4 | Modified information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 39.71 |
| PaDEL | 2D | MIC5 | Modified information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 39.71 |
| PaDEL | 2D | ZMIC0 | Z-modified information content index (neighborhood symmetry of 0-order) | Information Content descriptor | 103.72 |
| PaDEL | 2D | ZMIC1 | Z-modified information content index (neighborhood symmetry of 1-order) | Information Content descriptor | 45.88 |
| PaDEL | 2D | ZMIC2 | Z-modified information content index (neighborhood symmetry of 2-order) | Information Content descriptor | 30.87 |
| PaDEL | 2D | ZMIC3 | Z-modified information content index (neighborhood symmetry of 3-order) | Information Content descriptor | 29.24 |
| PaDEL | 2D | ZMIC4 | Z-modified information content index (neighborhood symmetry of 4-order) | Information Content descriptor | 29.11 |
| PaDEL | 2D | ZMIC5 | Z-modified information content index (neighborhood symmetry of 5-order) | Information Content descriptor | 29.11 |
| PaDEL | 2D | Kier1 | First kappa shape index | KappaShape Indices descriptor | 23.17 |
| PaDEL | 2D | Kier2 | Second kappa shape index | KappaShape Indices descriptor | 9.09 |
| PaDEL | 2D | Kier3 | Third kappa (κ) shape index | KappaShape Indices descriptor | 4.86 |
| PaDEL | 2D | nAtomLC | Number of atoms in the largest chain | Largest Chain descriptor | 5 |
| PaDEL | 2D | nAtomP | Number of atoms in the largest pi system | Largest Pi System descriptor | 13 |
| PaDEL | 2D | nAtomLAC | Number of atoms in the longest aliphatic chain | Longest Aliphatic Chain descriptor | 4 |
| PaDEL | 2D | MLogP | Mannhold LogP | Mannhold LogP descriptor | 3.66 |
| PaDEL | 2D | McGowan_Volume | McGowan characteristic volume | McGowan Volume descriptor | 3.1 |
| PaDEL | 2D | MDEC_11 | Molecular distance edge between all primary carbons | MDE descriptor | 1.19 |
| PaDEL | 2D | MDEC_12 | Molecular distance edge between all primary and secondary carbons | MDE descriptor | 5.46 |
| PaDEL | 2D | MDEC_13 | Molecular distance edge between all primary and tertiary carbons | MDE descriptor | 7.7 |
| PaDEL | 2D | MDEC_14 | Molecular distance edge between all primary and quaternary carbons | MDE descriptor | 1.51 |
| PaDEL | 2D | MDEC_22 | Molecular distance edge between all secondary carbons | MDE descriptor | 7.85 |
| PaDEL | 2D | MDEC_23 | Molecular distance edge between all secondary and tertiary carbons | MDE descriptor | 25.6 |
| PaDEL | 2D | MDEC_24 | Molecular distance edge between all secondary and quaternary carbons | MDE descriptor | 1.86 |
| PaDEL | 2D | MDEC_33 | Molecular distance edge between all tertiary carbons | MDE descriptor | 17.14 |
| PaDEL | 2D | MDEC_34 | Molecular distance edge between all tertiary and quaternary carbons | MDE descriptor | 2.37 |
| PaDEL | 2D | MDEC_44 | Molecular distance edge between all quaternary carbons | MDE descriptor | 0 |
| PaDEL | 2D | MDEO_11 | Molecular distance edge between all primary oxygens | MDE descriptor | 0.44 |
| PaDEL | 2D | MDEO_12 | Molecular distance edge between all primary and secondary oxygens | MDE descriptor | 1.12 |
| PaDEL | 2D | MDEO_22 | Molecular distance edge between all secondary oxygens | MDE descriptor | 0.25 |
| PaDEL | 2D | MDEN_11 | Molecular distance edge between all primary nitrogens | MDE descriptor | 0 |
| PaDEL | 2D | MDEN_12 | Molecular distance edge between all primary and secondary nitrogens | MDE descriptor | 0 |
| PaDEL | 2D | MDEN_13 | Molecular distance edge between all primary and tertiary niroqens | MDE descriptor | 0 |
| PaDEL | 2D | MDEN_22 | Molecular distance edge between all secondary nitroqens | MDE descriptor | 0 |
| PaDEL | 2D | MDEN_23 | Molecular distance edge between all secondary and tertiary nitrogens | MDE descriptor | 0 |
| PaDEL | 2D | MDEN_33 | Molecular distance edge between all tertiary nitrogens | MDE descriptor | 0 |
| PaDEL | 2D | MLFER_A | Overall or summation solute hydrogen bond acidity | MLFER descriptor | 0.67 |
| PaDEL | 2D | MLFER_BH | Overall or summation solute hydrogen bond basicity | MLFER descriptor | 1.34 |
| PaDEL | 2D | MLFER_BO | Overall or summation solute hydrogen bond basicity | MLFER descriptor | 1.33 |
| PaDEL | 2D | MLFER_S | Combined dipolarity/polarizability | MLFER descriptor | 2.22 |
| PaDEL | 2D | MLFER_E | Excessive molar refraction | MLFER descriptor | 2.49 |
| PaDEL | 2D | MLFER_L | Solute gas-hexadecane partition coefficient | MLFER descriptor | 15 |
| PaDEL | 2D | MPC2 | Molecular path count of order 2 | Path Count descriptor | 50 |
| PaDEL | 2D | MPC3 | Molecular path count of order 3 | Path Count descriptor | 66 |
| PaDEL | 2D | MPC4 | Molecular path count of order 4 | Path Count descriptor | 93 |
| PaDEL | 2D | MPC5 | Molecular path count of order 5 | Path Count descriptor | 134 |
| PaDEL | 2D | MPC6 | Molecular path count of order 6 | Path Count descriptor | 166 |
| PaDEL | 2D | MPC7 | Molecular path count of order 7 | Path Count descriptor | 196 |
| PaDEL | 2D | MPC8 | Molecular path count of order 8 | Path Count descriptor | 248 |
| PaDEL | 2D | MPC9 | Molecular path count of order 9 | Path Count descriptor | 269 |
| PaDEL | 2D | MPC10 | Molecular path count of order 10 | Path Count descriptor | 300 |
| PaDEL | 2D | TPC | Total path count (up to order 10) | Path Count descriptor | 1585 |
| PaDEL | 2D | piPC1 | Conventional bond order ID number of order 1 (ln(1+x) | Path Count descriptor | 3.76 |
| PaDEL | 2D | piPC2 | Conventional bond order ID number of order 2 (ln(1+x) | Path Count descriptor | 4.39 |
| PaDEL | 2D | piPC3 | Conventional bond order ID number of order 3 (ln(1+x) | Path Count descriptor | 4.95 |
| PaDEL | 2D | piPC4 | Conventional bond order ID number of order 4 (ln(1+x) | Path Count descriptor | 5.53 |
| PaDEL | 2D | piPC5 | Conventional bond order ID number of order 5 (ln(1+x) | Path Count descriptor | 6.15 |
| PaDEL | 2D | piPC6 | Conventional bond order ID number of order 6 (ln(1+x) | Path Count descriptor | 6.52 |
| PaDEL | 2D | piPC7 | Conventional bond order ID number of order 7 (ln(1+x) | Path Count descriptor | 6.89 |
| PaDEL | 2D | piPC8 | Conventional bond order ID number of order 8 (ln(1+x) | Path Count descriptor | 7.32 |
| PaDEL | 2D | piPC9 | Conventional bond order ID number of order 9 (ln(1+x) | Path Count descriptor | 7.61 |
| PaDEL | 2D | piPC10 | Conventional bond order ID number of order 10 (ln(1+x) | Path Count descriptor | 7.89 |
| PaDEL | 2D | TpiPC | Total conventional bond order (up to order 10) (ln(1+x)) | Path Count descriptor | 9.09 |
| PaDEL | 2D | R_TpiPCTPC | Ratio of total conventional bond order (up to order 10) with total path count (up to order 10) | Path Count descriptor | 5.6 |
| PaDEL | 2D | PetitjeanNumber | Petitjean number | Petitjean Number descriptor | 0.5 |
| PaDEL | 2D | nRing | Number of rings | Ring Count descriptor | 4 |
| PaDEL | 2D | n3Ring | Number of 3-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n4Ring | Number of 4-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n5Ring | Number of 5-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n6Ring | Number of 6-membered rings | Ring Count descriptor | 4 |
| PaDEL | 2D | n7Ring | Number of 7-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n8Ring | Number of 8-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n9Ring | Number of 9-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n10Ring | Number of 10-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n11Ring | Number of 11-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | n12Ring | Number of 12-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nG12Ring | Number of >12-membered rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nFRing | Number of fused rings | Ring Count descriptor | 3 |
| PaDEL | 2D | nF4Ring | Number of 4-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF5Ring | Number of 5-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF6Ring | Number of 6-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF7Ring | Number of 7-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF8Ring | Number of 8-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF9Ring | Number of 9-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF10Ring | Number of 10-membered fused rings | Ring Count descriptor | 2 |
| PaDEL | 2D | nF11Ring | Number of 11-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nF12Ring | Number of 12-membered fused rings | Ring Count descriptor | 0 |
| PaDEL | 2D | nFG12Ring | Number of >12-membered fused rings | Ring Count descriptor | 1 |
| PaDEL | 2D | nTRing | Number of rings (includes counts from fused rings) | Ring Count descriptor | 7 |
| PaDEL | 2D | nT4Ring | Number of 4-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT5Ring | Number of 5-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT6Ring | Number of 6-membered rings (includes counts from fused rings) | Ring Count descriptor | 4 |
| PaDEL | 2D | nT7Ring | Number of 7-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT8Ring | Number of 8-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT9Ring | Number of 9-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT10Ring | Number of 10-membered rings (includes counts from fused rings) | Ring Count descriptor | 2 |
| PaDEL | 2D | nT11Ring | Number of 11-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT12Ring | Number of 12-membered rings (includes counts from fused rings) | Ring Count descriptor | 0 |
| PaDEL | 2D | nTG12Ring | Number of >12-membered rings (includes counts from fused rings) | Ring Count descriptor | 1 |
| PaDEL | 2D | nHeteroRing | Number of rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 2 |
| PaDEL | 2D | n3HeteroRing | Number of 3-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n4HeteroRing | Number of 4-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n5HeteroRing | Number of 5-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n6HeteroRing | Number of 6-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 2 |
| PaDEL | 2D | n7HeteroRing | Number of 7-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n8HeteroRing | Number of 8-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n9HeteroRing | Number of 9-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n10HeteroRing | Number of 10-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n11HeteroRing | Number of 11-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | n12HeteroRing | Number of 12-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nG12HeteroRing | Number of >12-membered rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nFHeteroRing | Number of fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF4HeteroRing | Number of 4-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF5HeteroRing | Number of 5-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF6HeteroRing | Number of 6-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF7HeteroRing | Number of 7-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF8HeteroRing | Number of 8-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF9HeteroRing | Number of 9-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF10HeteroRing | Number of 10-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 2 |
| PaDEL | 2D | nF11HeteroRing | Number of 11-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nF12HeteroRing | Number of 12-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nFG12HeteroRing | Number of >12-membered fused rings containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 1 |
| PaDEL | 2D | nTHeteroRing | Number of rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or halogens) | Ring Count descriptor | 0 |
| PaDEL | 2D | nT4HeteroRing | Number of 4-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or | Ring Count descriptor | 0 |
| PaDEL | 2D | nT5HeteroRing | Number of 5-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or | Ring Count descriptor | 0 |
| PaDEL | 2D | nT6HeteroRing | Number of 6-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or | Ring Count descriptor | 2 |
| PaDEL | 2D | nT7HeteroRing | Number of 7-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or | Ring Count descriptor | 0 |
| PaDEL | 2D | nT8HeteroRing | Number of 8-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or | Ring Count descriptor | 0 |
| PaDEL | 2D | nT9HeteroRing | Number of 9-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, or | Ring Count descriptor | 0 |
| PaDEL | 2D | nT10HeteroRing | Number of 10-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, o | Ring Count descriptor | 2 |
| PaDEL | 2D | nT11HeteroRing | Number of 11-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, o | Ring Count descriptor | 0 |
| PaDEL | 2D | nT12HeteroRing | Number of 12-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, o | Ring Count descriptor | 0 |
| PaDEL | 2D | nTG12HeteroRing | Number of >12-membered rings (includes counts from fused rings) containing heteroatoms (N, O, P, S, | Ring Count descriptor | 1 |
| PaDEL | 2D | nRotB | Number of rotatable bonds, excluding terminal bonds | PaDEL Rotatable Bonds Count descriptor | 3 |
| PaDEL | 2D | RotBFrac | Fraction of rotatable bonds, excluding terminal bonds | PaDEL Rotatable Bonds Count descriptor | 0.09 |
| PaDEL | 2D | nRotBt | Number of rotatable bonds, including terminal bonds | PaDEL Rotatable Bonds Count descriptor | 9 |
| PaDEL | 2D | RotBtFrac | Fraction of rotatable bonds, including terminal bonds | PaDEL Rotatable Bonds Count descriptor | 0.27 |
| PaDEL | 2D | LipinskiFailures | Number failures of the Lipinski's Rule Of 5 | Rule Of Five descriptor | 0 |
| PaDEL | 2D | topoRadius | Topological radius (minimum atom eccentricity) | Topological descriptor | 7 |
| PaDEL | 2D | topoDiameter | Topological diameter (maximum atom eccentricity) | Topological descriptor | 14 |
| PaDEL | 2D | topoShape | Petitjean topological shape index | Topological descriptor | 1 |
| PaDEL | 2D | GGI1 | Topological charge index of order 1 | Topological Charge descriptor | 8 |
| PaDEL | 2D | GGI2 | Topological charge index of order 2 | Topological Charge descriptor | 4.22 |
| PaDEL | 2D | GGI3 | Topological charge index of order 3 | Topological Charge descriptor | 2.63 |
| PaDEL | 2D | GGI4 | Topological charge index of order 4 | Topological Charge descriptor | 2.9 |
| PaDEL | 2D | GGI5 | Topological charge index of order 5 | Topological Charge descriptor | 1.99 |
| PaDEL | 2D | GGI6 | Topological charge index of order 6 | Topological Charge descriptor | 1.17 |
| PaDEL | 2D | GGI7 | Topological charge index of order 7 | Topological Charge descriptor | 0.96 |
| PaDEL | 2D | GGI8 | Topological charge index of order 8 | Topological Charge descriptor | 0.43 |
| PaDEL | 2D | GGI9 | Topological charge index of order 9 | Topological Charge descriptor | 0.25 |
| PaDEL | 2D | GGI10 | Topological charge index of order 10 | Topological Charge descriptor | 0.14 |
| PaDEL | 2D | JGI1 | Mean topological charge index of order 1 | Topological Charge descriptor | 0.24 |
| PaDEL | 2D | JGI2 | Mean topological charge index of order 2 | Topological Charge descriptor | 0.08 |
| PaDEL | 2D | JGI3 | Mean topological charge index of order 3 | Topological Charge descriptor | 0.05 |
| PaDEL | 2D | JGI4 | Mean topological charge index of order 4 | Topological Charge descriptor | 0.05 |
| PaDEL | 2D | JGI5 | Mean topological charge index of order 5 | Topological Charge descriptor | 0.04 |
| PaDEL | 2D | JGI6 | Mean topological charge index of order 6 | Topological Charge descriptor | 0.02 |
| PaDEL | 2D | JGI7 | Mean topological charge index of order 7 | Topological Charge descriptor | 0.02 |
| PaDEL | 2D | JGI8 | Mean topological charge index of order 8 | Topological Charge descriptor | 0.01 |
| PaDEL | 2D | JGI9 | Mean topological charge index of order 9 | Topological Charge descriptor | 0.01 |
| PaDEL | 2D | JGI10 | Mean topological charge index of order 10 | Topological Charge descriptor | 0.01 |
| PaDEL | 2D | JGT | Global topological charge index | Topological Charge descriptor | 0.57 |
| PaDEL | 2D | SpMax_D | Leading eigenvalue from topological distance matrix | Topological Distance Matrix descriptor | 160.66 |
| PaDEL | 2D | SpDiam_D | Spectral diameter from topological distance matrix | Topological Distance Matrix descriptor | 227.38 |
| PaDEL | 2D | SpAD_D | Spectral absolute deviation from topological distance matrix | Topological Distance Matrix descriptor | 321.33 |
| PaDEL | 2D | SpMAD_D | Spectral mean absolute deviation from topological distance matrix | Topological Distance Matrix descriptor | 10.71 |
| PaDEL | 2D | EE_D | Estrada-like index from topological distance matrix | Topological Distance Matrix descriptor | 160.66 |
| PaDEL | 2D | VE1_D | Coefficient sum of the last eigenvector from topological distance matrix | Topological Distance Matrix descriptor | 0.15 |
| PaDEL | 2D | VE2_D | Average coefficient sum of the last eigenvector from topological distance matrix | Topological Distance Matrix descriptor | 0 |
| PaDEL | 2D | VE3_D | Logarithmic coefficient sum of the last eigenvector from topological distance matrix | Topological Distance Matrix descriptor | -5.77 |
| PaDEL | 2D | VR1_D | Randic-like eigenvector-based index from topological distance matrix | Topological Distance Matrix descriptor | 416.53 |
| PaDEL | 2D | VR2_D | Normalized Randic-like eigenvector-based index from topological distance matrix | Topological Distance Matrix descriptor | 13.88 |
| PaDEL | 2D | VR3_D | Logarithmic Randic-like eigenvector-based index from topological distance matrix | Topological Distance Matrix descriptor | 18.1 |
| PaDEL | 2D | TopoPSA | Topological polar surface area | TPSA descriptor | 75.99 |
| PaDEL | 2D | VABC | Van der Waals volume calculated using the method proposed in [Zhao, Yuan H. and Abraham, Michael H. | VABC descriptor | 389.95 |
| PaDEL | 2D | VAdjMat | Vertex adjacency information (magnitude) | VAdjMa descriptor | 5.91 |
| PaDEL | 2D | MWC2 | Molecular walk count of order 2 (ln(1+x) | WalkCount descriptor | 5.12 |
| PaDEL | 2D | MWC3 | Molecular walk count of order 3 (ln(1+x) | WalkCount descriptor | 5.99 |
| PaDEL | 2D | MWC4 | Molecular walk count of order 4 (ln(1+x) | WalkCount descriptor | 6.91 |
| PaDEL | 2D | MWC5 | Molecular walk count of order 5 (ln(1+x) | WalkCount descriptor | 7.82 |
| PaDEL | 2D | MWC6 | Molecular walk count of order 6 (ln(1+x) | WalkCount descriptor | 8.75 |
| PaDEL | 2D | MWC7 | Molecular walk count of order 7 (ln(1+x) | WalkCount descriptor | 9.66 |
| PaDEL | 2D | MWC8 | Molecular walk count of order 8 (ln(1+x) | WalkCount descriptor | 10.6 |
| PaDEL | 2D | MWC9 | Molecular walk count of order 9 (ln(1+x) | WalkCount descriptor | 11.53 |
| PaDEL | 2D | MWC10 | Molecular walk count of order 10 (ln(1+x) | WalkCount descriptor | 12.47 |
| PaDEL | 2D | TWC | Total walk count (up to order 10) | WalkCount descriptor | 141.85 |
| PaDEL | 2D | SRW2 | Self-returning walk count of order 2 (ln(1+x) | WalkCount descriptor | 4.2 |
| PaDEL | 2D | SRW3 | Self-returning walk count of order 3 (ln(1+x) | WalkCount descriptor | 0 |
| PaDEL | 2D | SRW4 | Self-returning walk count of order 4 (ln(1+x) | WalkCount descriptor | 5.59 |
| PaDEL | 2D | SRW5 | Self-returning walk count of order 5 (ln(1+x) | WalkCount descriptor | 0 |
| PaDEL | 2D | SRW6 | Self-returning walk count of order 6 (ln(1+x) | WalkCount descriptor | 7.16 |
| PaDEL | 2D | SRW7 | Self-returning walk count of order 7 (ln(1+x) | WalkCount descriptor | 0 |
| PaDEL | 2D | SRW8 | Self-returning walk count of order 8 (ln(1+x) | WalkCount descriptor | 8.84 |
| PaDEL | 2D | SRW9 | Self-returning walk count of order 9 (ln(1+x) | WalkCount descriptor | 0 |
| PaDEL | 2D | SRW10 | Self-returning walk count of order 10 (ln(1+x) | WalkCount descriptor | 10.57 |
| PaDEL | 2D | TSRW | Total self-return walk count (up to order 10) (ln(1+x)) | WalkCount descriptor | 10.77 |
| PaDEL | 2D | MW | Molecular weight | PaDEL Weight descriptor | 406.18 |
| PaDEL | 2D | AMW | Average molecular weight (Molecular weight / Total number of atoms) | PaDEL Weight descriptor | 7.25 |
| PaDEL | 2D | WTPT_1 | Molecular ID | PaDEL Weighted Path descriptor | 61.19 |
| PaDEL | 2D | WTPT_2 | Molecular ID / number of atoms | PaDEL Weighted Path descriptor | 2.04 |
| PaDEL | 2D | WTPT_3 | Sum of path lengths starting from heteroatoms | PaDEL Weighted Path descriptor | 14.03 |
| PaDEL | 2D | WTPT_4 | Sum of path lengths starting from oxygens | PaDEL Weighted Path descriptor | 14.03 |
| PaDEL | 2D | WTPT_5 | Sum of path lengths starting from nitrogens | PaDEL Weighted Path descriptor | 0 |
| PaDEL | 2D | WPATH | Weiner path number | Wiener Numbers descriptor | 2306 |
| PaDEL | 2D | WPOL | Weiner polarity number | Wiener Numbers descriptor | 54 |
| PaDEL | 2D | XLogP | XLogP | XLogP descriptor | 3.77 |
| PaDEL | 2D | Zagreb | Sum of the squares of atom degree over all heavy atoms i | Zagreb Index descriptor | 166 |
| PaDEL | 3D | TDB1u | 3D topological distance based autocorrelation - lag 1 / unweighted | Auto correlation 3D descriptor | 1.28 |
| PaDEL | 3D | TDB2u | 3D topological distance based autocorrelation - lag 2 / unweighted | Auto correlation 3D descriptor | 2.24 |
| PaDEL | 3D | TDB3u | 3D topological distance based autocorrelation - lag 3 / unweighted | Auto correlation 3D descriptor | 3.13 |
| PaDEL | 3D | TDB4u | 3D topological distance based autocorrelation - lag 4 / unweighted | Auto correlation 3D descriptor | 3.93 |
| PaDEL | 3D | TDB5u | 3D topological distance based autocorrelation - lag 5 / unweighted | Auto correlation 3D descriptor | 4.88 |
| PaDEL | 3D | TDB6u | 3D topological distance based autocorrelation - lag 6 / unweighted | Auto correlation 3D descriptor | 5.83 |
| PaDEL | 3D | TDB7u | 3D topological distance based autocorrelation - lag 7 / unweighted | Auto correlation 3D descriptor | 6.49 |
| PaDEL | 3D | TDB8u | 3D topological distance based autocorrelation - lag 8 / unweighted | Auto correlation 3D descriptor | 7.25 |
| PaDEL | 3D | TDB9u | 3D topological distance based autocorrelation - lag 9 / unweighted | Auto correlation 3D descriptor | 7.83 |
| PaDEL | 3D | TDB10u | 3D topological distance based autocorrelation - lag 10 / unweighted | Auto correlation 3D descriptor | 8.16 |
| PaDEL | 3D | TDB1m | 3D topological distance based autocorrelation - lag 1 / weighted by mass | Auto correlation 3D descriptor | 128.8 |
| PaDEL | 3D | TDB2m | 3D topological distance based autocorrelation - lag 2 / weighted by mass | Auto correlation 3D descriptor | 198.15 |
| PaDEL | 3D | TDB3m | 3D topological distance based autocorrelation - lag 3 / weighted by mass | Auto correlation 3D descriptor | 237.02 |
| PaDEL | 3D | TDB4m | 3D topological distance based autocorrelation - lag 4 / weighted by mass | Auto correlation 3D descriptor | 262.92 |
| PaDEL | 3D | TDB5m | 3D topological distance based autocorrelation - lag 5 / weighted by mass | Auto correlation 3D descriptor | 330.12 |
| PaDEL | 3D | TDB6m | 3D topological distance based autocorrelation - lag 6 / weighted by mass | Auto correlation 3D descriptor | 408.95 |
| PaDEL | 3D | TDB7m | 3D topological distance based autocorrelation - lag 7 / weighted by mass | Auto correlation 3D descriptor | 339.92 |
| PaDEL | 3D | TDB8m | 3D topological distance based autocorrelation - lag 8 / weighted by mass | Auto correlation 3D descriptor | 385.84 |
| PaDEL | 3D | TDB9m | 3D topological distance based autocorrelation - lag 9 / weighted by mass | Auto correlation 3D descriptor | 277.85 |
| PaDEL | 3D | TDB10m | 3D topological distance based autocorrelation - lag 10 / weighted by mass | Auto correlation 3D descriptor | 244.74 |
| PaDEL | 3D | TDB1v | 3D topological distance based autocorrelation - lag 1 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 372.09 |
| PaDEL | 3D | TDB2v | 3D topological distance based autocorrelation - lag 2 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 560.47 |
| PaDEL | 3D | TDB3v | 3D topological distance based autocorrelation - lag 3 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 696.56 |
| PaDEL | 3D | TDB4v | 3D topological distance based autocorrelation - lag 4 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 758.42 |
| PaDEL | 3D | TDB5v | 3D topological distance based autocorrelation - lag 5 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 996.74 |
| PaDEL | 3D | TDB6v | 3D topological distance based autocorrelation - lag 6 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 1200.62 |
| PaDEL | 3D | TDB7v | 3D topological distance based autocorrelation - lag 7 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 1135.69 |
| PaDEL | 3D | TDB8v | 3D topological distance based autocorrelation - lag 8 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 1247.05 |
| PaDEL | 3D | TDB9v | 3D topological distance based autocorrelation - lag 9 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 1131.15 |
| PaDEL | 3D | TDB10v | 3D topological distance based autocorrelation - lag 10 / weighted by van der Waals volumes | Auto correlation 3D descriptor | 935.49 |
| PaDEL | 3D | TDB1e | 3D topological distance based autocorrelation - lag 1 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 9.9 |
| PaDEL | 3D | TDB2e | 3D topological distance based autocorrelation - lag 2 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 17.26 |
| PaDEL | 3D | TDB3e | 3D topological distance based autocorrelation - lag 3 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 24.6 |
| PaDEL | 3D | TDB4e | 3D topological distance based autocorrelation - lag 4 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 30.44 |
| PaDEL | 3D | TDB5e | 3D topological distance based autocorrelation - lag 5 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 37.35 |
| PaDEL | 3D | TDB6e | 3D topological distance based autocorrelation - lag 6 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 45.76 |
| PaDEL | 3D | TDB7e | 3D topological distance based autocorrelation - lag 7 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 51.01 |
| PaDEL | 3D | TDB8e | 3D topological distance based autocorrelation - lag 8 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 54.51 |
| PaDEL | 3D | TDB9e | 3D topological distance based autocorrelation - lag 9 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 60.02 |
| PaDEL | 3D | TDB10e | 3D topological distance based autocorrelation - lag 10 / weighted by Sanderson electronegativities | Auto correlation 3D descriptor | 58.45 |
| PaDEL | 3D | TDB1p | 3D topological distance based autocorrelation - lag 1 / weighted by polarizabilities | Auto correlation 3D descriptor | 2.5 |
| PaDEL | 3D | TDB2p | 3D topological distance based autocorrelation - lag 2 / weighted by polarizabilities | Auto correlation 3D descriptor | 3.8 |
| PaDEL | 3D | TDB3p | 3D topological distance based autocorrelation - lag 3 / weighted by polarizabilities | Auto correlation 3D descriptor | 4.81 |
| PaDEL | 3D | TDB4p | 3D topological distance based autocorrelation - lag 4 / weighted by polarizabilities | Auto correlation 3D descriptor | 5.38 |
| PaDEL | 3D | TDB5p | 3D topological distance based autocorrelation - lag 5 / weighted by polarizabilities | Auto correlation 3D descriptor | 7.04 |
| PaDEL | 3D | TDB6p | 3D topological distance based autocorrelation - lag 6 / weighted by polarizabilities | Auto correlation 3D descriptor | 8.42 |
| PaDEL | 3D | TDB7p | 3D topological distance based autocorrelation - lag 7 / weighted by polarizabilities | Auto correlation 3D descriptor | 8.23 |
| PaDEL | 3D | TDB8p | 3D topological distance based autocorrelation - lag 8 / weighted by polarizabilities | Auto correlation 3D descriptor | 9.32 |
| PaDEL | 3D | TDB9p | 3D topological distance based autocorrelation - lag 9 / weighted by polarizabilities | Auto correlation 3D descriptor | 8.93 |
| PaDEL | 3D | TDB10p | 3D topological distance based autocorrelation - lag 10 / weighted by polarizabilities | Auto correlation 3D descriptor | 7.92 |
| PaDEL | 3D | TDB1i | 3D topological distance based autocorrelation - lag 1 / weighted by first ionization potential | Auto correlation 3D descriptor | 179.66 |
| PaDEL | 3D | TDB2i | 3D topological distance based autocorrelation - lag 2 / weighted by first ionization potential | Auto correlation 3D descriptor | 329.05 |
| PaDEL | 3D | TDB3i | 3D topological distance based autocorrelation - lag 3 / weighted by first ionization potential | Auto correlation 3D descriptor | 469.43 |
| PaDEL | 3D | TDB4i | 3D topological distance based autocorrelation - lag 4 / weighted by first ionization potential | Auto correlation 3D descriptor | 612.14 |
| PaDEL | 3D | TDB5i | 3D topological distance based autocorrelation - lag 5 / weighted by first ionization potential | Auto correlation 3D descriptor | 750.23 |
| PaDEL | 3D | TDB6i | 3D topological distance based autocorrelation - lag 6 / weighted by first ionization potential | Auto correlation 3D descriptor | 885 |
| PaDEL | 3D | TDB7i | 3D topological distance based autocorrelation - lag 7 / weighted by first ionization potential | Auto correlation 3D descriptor | 1031.34 |
| PaDEL | 3D | TDB8i | 3D topological distance based autocorrelation - lag 8 / weighted by first ionization potential | Auto correlation 3D descriptor | 1130.21 |
| PaDEL | 3D | TDB9i | 3D topological distance based autocorrelation - lag 9 / weighted by first ionization potential | Auto correlation 3D descriptor | 1249.92 |
| PaDEL | 3D | TDB10i | 3D topological distance based autocorrelation - lag 10 / weighted by first ionization potential | Auto correlation 3D descriptor | 1357.17 |
| PaDEL | 3D | TDB1s | 3D topological distance based autocorrelation - lag 1 / weighted by I-state | Auto correlation 3D descriptor | 4.13 |
| PaDEL | 3D | TDB2s | 3D topological distance based autocorrelation - lag 2 / weighted by I-state | Auto correlation 3D descriptor | 7.01 |
| PaDEL | 3D | TDB3s | 3D topological distance based autocorrelation - lag 3 / weighted by I-state | Auto correlation 3D descriptor | 10.36 |
| PaDEL | 3D | TDB4s | 3D topological distance based autocorrelation - lag 4 / weighted by I-state | Auto correlation 3D descriptor | 13.33 |
| PaDEL | 3D | TDB5s | 3D topological distance based autocorrelation - lag 5 / weighted by I-state | Auto correlation 3D descriptor | 14.45 |
| PaDEL | 3D | TDB6s | 3D topological distance based autocorrelation - lag 6 / weighted by I-state | Auto correlation 3D descriptor | 21.71 |
| PaDEL | 3D | TDB7s | 3D topological distance based autocorrelation - lag 7 / weighted by I-state | Auto correlation 3D descriptor | 20.87 |
| PaDEL | 3D | TDB8s | 3D topological distance based autocorrelation - lag 8 / weighted by I-state | Auto correlation 3D descriptor | 22.58 |
| PaDEL | 3D | TDB9s | 3D topological distance based autocorrelation - lag 9 / weighted by I-state | Auto correlation 3D descriptor | 22.83 |
| PaDEL | 3D | TDB10s | 3D topological distance based autocorrelation - lag 10 / weighted by I-state | Auto correlation 3D descriptor | 19.03 |
| PaDEL | 3D | TDB1r | 3D topological distance based autocorrelation - lag 1 / weighted by covalent radius | Auto correlation 3D descriptor | 0.55 |
| PaDEL | 3D | TDB2r | 3D topological distance based autocorrelation - lag 2 / weighted by covalent radius | Auto correlation 3D descriptor | 0.86 |
| PaDEL | 3D | TDB3r | 3D topological distance based autocorrelation - lag 3 / weighted by covalent radius | Auto correlation 3D descriptor | 1.12 |
| PaDEL | 3D | TDB4r | 3D topological distance based autocorrelation - lag 4 / weighted by covalent radius | Auto correlation 3D descriptor | 1.25 |
| PaDEL | 3D | TDB5r | 3D topological distance based autocorrelation - lag 5 / weighted by covalent radius | Auto correlation 3D descriptor | 1.61 |
| PaDEL | 3D | TDB6r | 3D topological distance based autocorrelation - lag 6 / weighted by covalent radius | Auto correlation 3D descriptor | 1.98 |
| PaDEL | 3D | TDB7r | 3D topological distance based autocorrelation - lag 7 / weighted by covalent radius | Auto correlation 3D descriptor | 1.94 |
| PaDEL | 3D | TDB8r | 3D topological distance based autocorrelation - lag 8 / weighted by covalent radius | Auto correlation 3D descriptor | 2.14 |
| PaDEL | 3D | TDB9r | 3D topological distance based autocorrelation - lag 9 / weighted by covalent radius | Auto correlation 3D descriptor | 2.08 |
| PaDEL | 3D | TDB10r | 3D topological distance based autocorrelation - lag 10 / weighted by covalent radius | Auto correlation 3D descriptor | 1.78 |
| PaDEL | 3D | PPSA_1 | Partial positive surface area -- sum of surface area on positive parts of molecule | CPSA descriptor | 551.65 |
| PaDEL | 3D | PPSA_2 | Partial positive surface area * total positive charge on the molecule | CPSA descriptor | 1265.13 |
| PaDEL | 3D | PPSA_3 | Charge weighted partial positive surface area | CPSA descriptor | 35.77 |
| PaDEL | 3D | PNSA_1 | Partial negative surface area -- sum of surface area on negative parts of molecule | CPSA descriptor | 196.36 |
| PaDEL | 3D | PNSA_2 | Partial negative surface area * total negative charge on the molecule | CPSA descriptor | -450.31 |
| PaDEL | 3D | PNSA_3 | Charge weighted partial negative surface area | CPSA descriptor | -36.46 |
| PaDEL | 3D | DPSA_1 | Difference of PPSA-1 and PNSA-1 | CPSA descriptor | 355.3 |
| PaDEL | 3D | DPSA_2 | Difference of FPSA-2 and PNSA-2 | CPSA descriptor | 1715.44 |
| PaDEL | 3D | DPSA_3 | Difference of PPSA-3 and PNSA-3 | CPSA descriptor | 72.23 |
| PaDEL | 3D | FPSA_1 | PPSA-1 / total molecular surface area | CPSA descriptor | 0.74 |
| PaDEL | 3D | FPSA_2 | PPSA-2 / total molecular surface area | CPSA descriptor | 1.69 |
| PaDEL | 3D | FPSA_3 | PPSA-3 / total molecular surface area | CPSA descriptor | 0.05 |
| PaDEL | 3D | FNSA_1 | PNSA-1 / total molecular surface area | CPSA descriptor | 0.26 |
| PaDEL | 3D | FNSA_2 | PNSA-2 / total molecular surface area | CPSA descriptor | -0.6 |
| PaDEL | 3D | FNSA_3 | PNSA-3 / total molecular surface area | CPSA descriptor | -0.05 |
| PaDEL | 3D | WPSA_1 | PPSA-1 * total molecular surface area / 1000 | CPSA descriptor | 412.64 |
| PaDEL | 3D | WPSA_2 | PPSA-2 * total molecular surface area /1000 | CPSA descriptor | 946.33 |
| PaDEL | 3D | WPSA_3 | PPSA-3 * total molecular surface area / 1000 | CPSA descriptor | 26.76 |
| PaDEL | 3D | WNSA_1 | PNSA-1 * total molecular surface area /1000 | CPSA descriptor | 146.88 |
| PaDEL | 3D | WNSA_2 | PNSA-2 * total molecular surface area / 1000 | CPSA descriptor | -336.84 |
| PaDEL | 3D | WNSA_3 | PNSA-3 * total molecular surface area / 1000 | CPSA descriptor | -27.27 |
| PaDEL | 3D | RPCG | Relative positive charge -- most positive charge / total positive charge | CPSA descriptor | 0.1 |
| PaDEL | 3D | RNCG | Relative negative charge -- most negative charge / total negative charge | CPSA descriptor | 0.16 |
| PaDEL | 3D | RPCS | Relative positive charge surface area -- most positive surface area * RPCG | CPSA descriptor | 2.87 |
| PaDEL | 3D | RNCS | Relative negative charge surface area -- most negative surface area * RNCG | CPSA descriptor | 5.63 |
| PaDEL | 3D | THSA | Sum of solvent accessible surface areas of atoms with absolute value of partial charges less than 0. | CPSA descriptor | 601.44 |
| PaDEL | 3D | TPSA | Sum of solvent accessible surface areas of atoms with absolute value of partial charges greater than | CPSA descriptor | 146.57 |
| PaDEL | 3D | RHSA | THSA / total molecular surface area | CPSA descriptor | 0.8 |
| PaDEL | 3D | RPSA | TPSA / total molecular surface area | CPSA descriptor | 0.2 |
| PaDEL | 3D | GRAV_1 | Gravitational index of heavy atoms | Gravitational Index descriptor | 2533.05 |
| PaDEL | 3D | GRAV_2 | Square root of gravitational index of heavy atoms | Gravitational Index descriptor | 50.33 |
| PaDEL | 3D | GRAV_3 | Cube root of gravitational index of heavy atoms | Gravitational Index descriptor | 13.63 |
| PaDEL | 3D | GRAVH_1 | Gravitational index - hydrogens included | Gravitational Index descriptor | 2808.1 |
| PaDEL | 3D | GRAVH_2 | Square root of hydrogen-included gravitational index | Gravitational Index descriptor | 52.99 |
| PaDEL | 3D | GRAVH_3 | Cube root of hydrogen-included gravitational index | Gravitational Index descriptor | 14.11 |
| PaDEL | 3D | GRAV_4 | Gravitational index of all pairs of atoms (not just bonded pairs) | Gravitational Index descriptor | 6050.51 |
| PaDEL | 3D | GRAV_5 | Square root of gravitational index of all pairs of atoms (not just bonded pairs) | Gravitational Index descriptor | 77.79 |
| PaDEL | 3D | GRAV_6 | Cube root of gravitational index of all pairs of atoms (not just bonded pairs) | Gravitational Index descriptor | 18.22 |
| PaDEL | 3D | LOBMAX | The maximum L/B ratio | Length Over Breadth descriptor | 1.84 |
| PaDEL | 3D | LOBMIN | The L/B ratio for the rotation that results in the minimum area | Length Over Breadth descriptor | 1.84 |
| PaDEL | 3D | MOMI_X | Moment of inertia along X axis | Moment Of Inertia descriptor | 7787.98 |
| PaDEL | 3D | MOMI_Y | Moment of inertia along Y axis | Moment Of Inertia descriptor | 6608.59 |
| PaDEL | 3D | MOMI_Z | Moment of inertia along Z axis | Moment Of Inertia descriptor | 1668.08 |
| PaDEL | 3D | MOMI_XY | X/Y | Moment Of Inertia descriptor | 1.18 |
| PaDEL | 3D | MOMI_XZ | X/Z | Moment Of Inertia descriptor | 4.67 |
| PaDEL | 3D | MOMI_YZ | Y/Z | Moment Of Inertia descriptor | 3.96 |
| PaDEL | 3D | MOMI_R | Radius of gyration | Moment Of Inertia descriptor | 8.26 |
| PaDEL | 3D | geomRadius | Geometrical radius (minimum geometric eccentricity) | PaDEL Petitjean Shape Index descriptor | 8.11 |
| PaDEL | 3D | geomDiameter | Geometrical diameter (maximum geometric eccentricity) | PaDEL Petitjean Shape Index descriptor | 15.5 |
| PaDEL | 3D | geomShape | Petitjean geometric shape index | PaDEL Petitjean Shape Index descriptor | 0.91 |
| PaDEL | 3D | RDF10u | Radial distribution function - 010 / unweighted | RDF descriptor | 11.97 |
| PaDEL | 3D | RDF15u | Radial distribution function - 015 / unweighted | RDF descriptor | 17.86 |
| PaDEL | 3D | RDF20u | Radial distribution function - 020 / unweighted | RDF descriptor | 7.27 |
| PaDEL | 3D | RDF25u | Radial distribution function - 025 / unweighted | RDF descriptor | 47.76 |
| PaDEL | 3D | RDF30u | Radial distribution function - 030 / unweighted | RDF descriptor | 8.58 |
| PaDEL | 3D | RDF35u | Radial distribution function - 035 / unweighted | RDF descriptor | 34.52 |
| PaDEL | 3D | RDF40u | Radial distribution function - 040 / unweighted | RDF descriptor | 24.25 |
| PaDEL | 3D | RDF45u | Radial distribution function - 045 / unweighted | RDF descriptor | 34.76 |
| PaDEL | 3D | RDF50u | Radial distribution function - 050 / unweighted | RDF descriptor | 40.82 |
| PaDEL | 3D | RDF55u | Radial distribution function - 055 / unweighted | RDF descriptor | 25.47 |
| PaDEL | 3D | RDF60u | Radial distribution function - 060 / unweighted | RDF descriptor | 39.44 |
| PaDEL | 3D | RDF65u | Radial distribution function - 065 / unweighted | RDF descriptor | 28.54 |
| PaDEL | 3D | RDF70u | Radial distribution function - 070 / unweighted | RDF descriptor | 28.96 |
| PaDEL | 3D | RDF75u | Radial distribution function - 075 / unweighted | RDF descriptor | 21.19 |
| PaDEL | 3D | RDF80u | Radial distribution function - 080 / unweighted | RDF descriptor | 18.39 |
| PaDEL | 3D | RDF85u | Radial distribution function - 085 / unweighted | RDF descriptor | 18.34 |
| PaDEL | 3D | RDF90u | Radial distribution function - 090 / unweighted | RDF descriptor | 14.49 |
| PaDEL | 3D | RDF95u | Radial distribution function - 095 / unweighted | RDF descriptor | 14.83 |
| PaDEL | 3D | RDF100u | Radial distribution function - 100 / unweighted | RDF descriptor | 12.45 |
| PaDEL | 3D | RDF105u | Radial distribution function - 105 / unweighted | RDF descriptor | 17.72 |
| PaDEL | 3D | RDF110u | Radial distribution function - 110 / unweighted | RDF descriptor | 10.76 |
| PaDEL | 3D | RDF115u | Radial distribution function - 115 / unweighted | RDF descriptor | 7.13 |
| PaDEL | 3D | RDF120u | Radial distribution function - 120 / unweighted | RDF descriptor | 8.34 |
| PaDEL | 3D | RDF125u | Radial distribution function - 125 / unweighted | RDF descriptor | 7.19 |
| PaDEL | 3D | RDF130u | Radial distribution function - 130 / unweighted | RDF descriptor | 5.07 |
| PaDEL | 3D | RDF135u | Radial distribution function - 135 / unweighted | RDF descriptor | 3.19 |
| PaDEL | 3D | RDF140u | Radial distribution function - 140 / unweighted | RDF descriptor | 2.18 |
| PaDEL | 3D | RDF145u | Radial distribution function - 145 / unweighted | RDF descriptor | 1.79 |
| PaDEL | 3D | RDF150u | Radial distribution function - 150 / unweighted | RDF descriptor | 1.7 |
| PaDEL | 3D | RDF155u | Radial distribution function - 155 / unweighted | RDF descriptor | 1.97 |
| PaDEL | 3D | RDF10m | Radial distribution function - 010 / weighted by relative mass | RDF descriptor | 1.06 |
| PaDEL | 3D | RDF15m | Radial distribution function - 015 / weighted by relative mass | RDF descriptor | 18.52 |
| PaDEL | 3D | RDF20m | Radial distribution function - 020 / weighted by relative mass | RDF descriptor | 0.55 |
| PaDEL | 3D | RDF25m | Radial distribution function - 025 / weighted by relative mass | RDF descriptor | 35.81 |
| PaDEL | 3D | RDF30m | Radial distribution function - 030 / weighted by relative mass | RDF descriptor | 2.48 |
| PaDEL | 3D | RDF35m | Radial distribution function - 035 / weighted by relative mass | RDF descriptor | 10.06 |
| PaDEL | 3D | RDF40m | Radial distribution function - 040 / weighted by relative mass | RDF descriptor | 7.87 |
| PaDEL | 3D | RDF45m | Radial distribution function - 045 / weighted by relative mass | RDF descriptor | 9.61 |
| PaDEL | 3D | RDF50m | Radial distribution function - 050 / weighted by relative mass | RDF descriptor | 19.75 |
| PaDEL | 3D | RDF55m | Radial distribution function - 055 / weighted by relative mass | RDF descriptor | 8.67 |
| PaDEL | 3D | RDF60m | Radial distribution function - 060 / weighted by relative mass | RDF descriptor | 16.31 |
| PaDEL | 3D | RDF65m | Radial distribution function - 065 / weighted by relative mass | RDF descriptor | 11.38 |
| PaDEL | 3D | RDF70m | Radial distribution function - 070 / weighted by relative mass | RDF descriptor | 10.67 |
| PaDEL | 3D | RDF75m | Radial distribution function - 075 / weighted by relative mass | RDF descriptor | 6.64 |
| PaDEL | 3D | RDF80m | Radial distribution function - 080 / weighted by relative mass | RDF descriptor | 4.23 |
| PaDEL | 3D | RDF85m | Radial distribution function - 085 / weighted by relative mass | RDF descriptor | 7.35 |
| PaDEL | 3D | RDF90m | Radial distribution function - 090 / weighted by relative mass | RDF descriptor | 3.68 |
| PaDEL | 3D | RDF95m | Radial distribution function - 095 / weighted by relative mass | RDF descriptor | 5.64 |
| PaDEL | 3D | RDF100m | Radial distribution function - 100 / weighted by relative mass | RDF descriptor | 1.97 |
| PaDEL | 3D | RDF105m | Radial distribution function - 105 / weighted by relative mass | RDF descriptor | 5.14 |
| PaDEL | 3D | RDF110m | Radial distribution function - 110 / weighted by relative mass | RDF descriptor | 1.88 |
| PaDEL | 3D | RDF115m | Radial distribution function - 115 / weighted by relative mass | RDF descriptor | 3.05 |
| PaDEL | 3D | RDF120m | Radial distribution function - 120 / weighted by relative mass | RDF descriptor | 2.32 |
| PaDEL | 3D | RDF125m | Radial distribution function - 125 / weighted by relative mass | RDF descriptor | 1.36 |
| PaDEL | 3D | RDF130m | Radial distribution function - 130 / weighted by relative mass | RDF descriptor | 0.81 |
| PaDEL | 3D | RDF135m | Radial distribution function - 135 / weighted by relative mass | RDF descriptor | 0.73 |
| PaDEL | 3D | RDF140m | Radial distribution function - 140 / weighted by relative mass | RDF descriptor | 0.75 |
| PaDEL | 3D | RDF145m | Radial distribution function - 145 / weighted by relative mass | RDF descriptor | 0.99 |
| PaDEL | 3D | RDF150m | Radial distribution function - 150 / weighted by relative mass | RDF descriptor | 0.08 |
| PaDEL | 3D | RDF155m | Radial distribution function - 155 / weighted by relative mass | RDF descriptor | 0.12 |
| PaDEL | 3D | RDF10v | Radial distribution function - 010 / weighted by relative van der Waals volumes | RDF descriptor | 3.1 |
| PaDEL | 3D | RDF15v | Radial distribution function - 015 / weighted by relative van der Waals volumes | RDF descriptor | 17.26 |
| PaDEL | 3D | RDF20v | Radial distribution function - 020 / weighted by relative van der Waals volumes | RDF descriptor | 1.71 |
| PaDEL | 3D | RDF25v | Radial distribution function - 025 / weighted by relative van der Waals volumes | RDF descriptor | 32.79 |
| PaDEL | 3D | RDF30v | Radial distribution function - 030 / weighted by relative van der Waals volumes | RDF descriptor | 2.76 |
| PaDEL | 3D | RDF35v | Radial distribution function - 035 / weighted by relative van der Waals volumes | RDF descriptor | 12.9 |
| PaDEL | 3D | RDF40v | Radial distribution function - 040 / weighted by relative van der Waals volumes | RDF descriptor | 9.49 |
| PaDEL | 3D | RDF45v | Radial distribution function - 045 / weighted by relative van der Waals volumes | RDF descriptor | 13.46 |
| PaDEL | 3D | RDF50v | Radial distribution function - 050 / weighted by relative van der Waals volumes | RDF descriptor | 20.61 |
| PaDEL | 3D | RDF55v | Radial distribution function - 055 / weighted by relative van der Waals volumes | RDF descriptor | 10.28 |
| PaDEL | 3D | RDF60v | Radial distribution function - 060 / weighted by relative van der Waals volumes | RDF descriptor | 17.45 |
| PaDEL | 3D | RDF65v | Radial distribution function - 065 / weighted by relative van der Waals volumes | RDF descriptor | 12.33 |
| PaDEL | 3D | RDF70v | Radial distribution function - 070 / weighted by relative van der Waals volumes | RDF descriptor | 12.86 |
| PaDEL | 3D | RDF75v | Radial distribution function - 075 / weighted by relative van der Waals volumes | RDF descriptor | 7.39 |
| PaDEL | 3D | RDF80v | Radial distribution function - 080 / weighted by relative van der Waals volumes | RDF descriptor | 6.33 |
| PaDEL | 3D | RDF85v | Radial distribution function - 085 / weighted by relative van der Waals volumes | RDF descriptor | 8.12 |
| PaDEL | 3D | RDF90v | Radial distribution function - 090 / weighted by relative van der Waals volumes | RDF descriptor | 5.02 |
| PaDEL | 3D | RDF95v | Radial distribution function - 095 / weighted by relative van der Waals volumes | RDF descriptor | 6.45 |
| PaDEL | 3D | RDF100v | Radial distribution function - 100 / weighted by relative van der Waals volumes | RDF descriptor | 3.65 |
| PaDEL | 3D | RDF105v | Radial distribution function - 105 / weighted by relative van der Waals volumes | RDF descriptor | 6.06 |
| PaDEL | 3D | RDF110v | Radial distribution function - 110 / weighted by relative van der Waals volumes | RDF descriptor | 2.93 |
| PaDEL | 3D | RDF115v | Radial distribution function - 115 / weighted by relative van der Waals volumes | RDF descriptor | 2.64 |
| PaDEL | 3D | RDF120v | Radial distribution function - 120 / weighted by relative van der Waals volumes | RDF descriptor | 2.98 |
| PaDEL | 3D | RDF125v | Radial distribution function - 125 / weighted by relative van der Waals volumes | RDF descriptor | 1.71 |
| PaDEL | 3D | RDF130v | Radial distribution function - 130 / weighted by relative van der Waals volumes | RDF descriptor | 1.59 |
| PaDEL | 3D | RDF135v | Radial distribution function - 135 / weighted by relative van der Waals volumes | RDF descriptor | 0.85 |
| PaDEL | 3D | RDF140v | Radial distribution function - 140 / weighted by relative van der Waals volumes | RDF descriptor | 0.74 |
| PaDEL | 3D | RDF145v | Radial distribution function - 145 / weighted by relative van der Waals volumes | RDF descriptor | 0.73 |
| PaDEL | 3D | RDF150v | Radial distribution function - 150 / weighted by relative van der Waals volumes | RDF descriptor | 0.21 |
| PaDEL | 3D | RDF155v | Radial distribution function - 155 / weighted by relative van der Waals volumes | RDF descriptor | 0.26 |
| PaDEL | 3D | RDF10e | Radial distribution function - 010 / weighted by relative Sanderson electronegativities | RDF descriptor | 11.87 |
| PaDEL | 3D | RDF15e | Radial distribution function - 015 / weighted by relative Sanderson electronegativities | RDF descriptor | 18.54 |
| PaDEL | 3D | RDF20e | Radial distribution function - 020 / weighted by relative Sanderson electronegativities | RDF descriptor | 7.05 |
| PaDEL | 3D | RDF25e | Radial distribution function - 025 / weighted by relative Sanderson electronegativities | RDF descriptor | 50.16 |
| PaDEL | 3D | RDF30e | Radial distribution function - 030 / weighted by relative Sanderson electronegativities | RDF descriptor | 8.32 |
| PaDEL | 3D | RDF35e | Radial distribution function - 035 / weighted by relative Sanderson electronegativities | RDF descriptor | 34.63 |
| PaDEL | 3D | RDF40e | Radial distribution function - 040 / weighted by relative Sanderson electronegativities | RDF descriptor | 24.08 |
| PaDEL | 3D | RDF45e | Radial distribution function - 045 / weighted by relative Sanderson electronegativities | RDF descriptor | 34.05 |
| PaDEL | 3D | RDF50e | Radial distribution function - 050 / weighted by relative Sanderson electronegativities | RDF descriptor | 41.43 |
| PaDEL | 3D | RDF55e | Radial distribution function - 055 / weighted by relative Sanderson electronegativities | RDF descriptor | 25.74 |
| PaDEL | 3D | RDF60e | Radial distribution function - 060 / weighted by relative Sanderson electronegativities | RDF descriptor | 40.01 |
| PaDEL | 3D | RDF65e | Radial distribution function - 065 / weighted by relative Sanderson electronegativities | RDF descriptor | 28.85 |
| PaDEL | 3D | RDF70e | Radial distribution function - 070 / weighted by relative Sanderson electronegativities | RDF descriptor | 29.02 |
| PaDEL | 3D | RDF75e | Radial distribution function - 075 / weighted by relative Sanderson electronegativities | RDF descriptor | 21.19 |
| PaDEL | 3D | RDF80e | Radial distribution function - 080 / weighted by relative Sanderson electronegativities | RDF descriptor | 18.08 |
| PaDEL | 3D | RDF85e | Radial distribution function - 085 / weighted by relative Sanderson electronegativities | RDF descriptor | 18.51 |
| PaDEL | 3D | RDF90e | Radial distribution function - 090 / weighted by relative Sanderson electronegativities | RDF descriptor | 14.3 |
| PaDEL | 3D | RDF95e | Radial distribution function - 095 / weighted by relative Sanderson electronegativities | RDF descriptor | 14.55 |
| PaDEL | 3D | RDF100e | Radial distribution function - 100 / weighted by relative Sanderson electronegativities | RDF descriptor | 11.72 |
| PaDEL | 3D | RDF105e | Radial distribution function - 105 / weighted by relative Sanderson electronegativities | RDF descriptor | 17.05 |
| PaDEL | 3D | RDF110e | Radial distribution function - 110 / weighted by relative Sanderson electronegativities | RDF descriptor | 10.7 |
| PaDEL | 3D | RDF115e | Radial distribution function - 115 / weighted by relative Sanderson electronegativities | RDF descriptor | 7.47 |
| PaDEL | 3D | RDF120e | Radial distribution function - 120 / weighted by relative Sanderson electronegativities | RDF descriptor | 8.01 |
| PaDEL | 3D | RDF125e | Radial distribution function - 125 / weighted by relative Sanderson electronegativities | RDF descriptor | 7.59 |
| PaDEL | 3D | RDF130e | Radial distribution function - 130 / weighted by relative Sanderson electronegativities | RDF descriptor | 4.78 |
| PaDEL | 3D | RDF135e | Radial distribution function - 135 / weighted by relative Sanderson electronegativities | RDF descriptor | 3.43 |
| PaDEL | 3D | RDF140e | Radial distribution function - 140 / weighted by relative Sanderson electronegativities | RDF descriptor | 2.23 |
| PaDEL | 3D | RDF145e | Radial distribution function - 145 / weighted by relative Sanderson electronegativities | RDF descriptor | 2.05 |
| PaDEL | 3D | RDF150e | Radial distribution function - 150 / weighted by relative Sanderson electronegativities | RDF descriptor | 1.77 |
| PaDEL | 3D | RDF155e | Radial distribution function - 155 / weighted by relative Sanderson electronegativities | RDF descriptor | 2.12 |
| PaDEL | 3D | RDF10p | Radial distribution function - 010 / weighted by relative polarizabilities | RDF descriptor | 4.4 |
| PaDEL | 3D | RDF15p | Radial distribution function - 015 / weighted by relative polarizabilities | RDF descriptor | 16.78 |
| PaDEL | 3D | RDF20p | Radial distribution function - 020 / weighted by relative polarizabilities | RDF descriptor | 2.5 |
| PaDEL | 3D | RDF25p | Radial distribution function - 025 / weighted by relative polarizabilities | RDF descriptor | 32.29 |
| PaDEL | 3D | RDF30p | Radial distribution function - 030 / weighted by relative polarizabilities | RDF descriptor | 3.24 |
| PaDEL | 3D | RDF35p | Radial distribution function - 035 / weighted by relative polarizabilities | RDF descriptor | 15.24 |
| PaDEL | 3D | RDF40p | Radial distribution function - 040 / weighted by relative polarizabilities | RDF descriptor | 11.08 |
| PaDEL | 3D | RDF45p | Radial distribution function - 045 / weighted by relative polarizabilities | RDF descriptor | 16.26 |
| PaDEL | 3D | RDF50p | Radial distribution function - 050 / weighted by relative polarizabilities | RDF descriptor | 22.16 |
| PaDEL | 3D | RDF55p | Radial distribution function - 055 / weighted by relative polarizabilities | RDF descriptor | 11.67 |
| PaDEL | 3D | RDF60p | Radial distribution function - 060 / weighted by relative polarizabilities | RDF descriptor | 19.19 |
| PaDEL | 3D | RDF65p | Radial distribution function - 065 / weighted by relative polarizabilities | RDF descriptor | 13.59 |
| PaDEL | 3D | RDF70p | Radial distribution function - 070 / weighted by relative polarizabilities | RDF descriptor | 14.55 |
| PaDEL | 3D | RDF75p | Radial distribution function - 075 / weighted by relative polarizabilities | RDF descriptor | 8.44 |
| PaDEL | 3D | RDF80p | Radial distribution function - 080 / weighted by relative polarizabilities | RDF descriptor | 7.88 |
| PaDEL | 3D | RDF85p | Radial distribution function - 085 / weighted by relative polarizabilities | RDF descriptor | 9.04 |
| PaDEL | 3D | RDF90p | Radial distribution function - 090 / weighted by relative polarizabilities | RDF descriptor | 6.01 |
| PaDEL | 3D | RDF95p | Radial distribution function - 095 / weighted by relative polarizabilities | RDF descriptor | 7.29 |
| PaDEL | 3D | RDF100p | Radial distribution function - 100 / weighted by relative polarizabilities | RDF descriptor | 4.93 |
| PaDEL | 3D | RDF105p | Radial distribution function - 105 / weighted by relative polarizabilities | RDF descriptor | 7.29 |
| PaDEL | 3D | RDF110p | Radial distribution function - 110 / weighted by relative polarizabilities | RDF descriptor | 3.76 |
| PaDEL | 3D | RDF115p | Radial distribution function - 115 / weighted by relative polarizabilities | RDF descriptor | 2.77 |
| PaDEL | 3D | RDF120p | Radial distribution function - 120 / weighted by relative polarizabilities | RDF descriptor | 3.61 |
| PaDEL | 3D | RDF125p | Radial distribution function - 125 / weighted by relative polarizabilities | RDF descriptor | 1.96 |
| PaDEL | 3D | RDF130p | Radial distribution function - 130 / weighted by relative polarizabilities | RDF descriptor | 2.15 |
| PaDEL | 3D | RDF135p | Radial distribution function - 135 / weighted by relative polarizabilities | RDF descriptor | 0.97 |
| PaDEL | 3D | RDF140p | Radial distribution function - 140 / weighted by relative polarizabilities | RDF descriptor | 0.83 |
| PaDEL | 3D | RDF145p | Radial distribution function - 145 / weighted by relative polarizabilities | RDF descriptor | 0.66 |
| PaDEL | 3D | RDF150p | Radial distribution function - 150 / weighted by relative polarizabilities | RDF descriptor | 0.29 |
| PaDEL | 3D | RDF155p | Radial distribution function - 155 / weighted by relative polarizabilities | RDF descriptor | 0.35 |
| PaDEL | 3D | RDF10i | Radial distribution function - 010 / weighted by relative first ionization potential | RDF descriptor | 14.92 |
| PaDEL | 3D | RDF15i | Radial distribution function - 015 / weighted by relative first ionization potential | RDF descriptor | 18.3 |
| PaDEL | 3D | RDF20i | Radial distribution function - 020 / weighted by relative first ionization potential | RDF descriptor | 9.23 |
| PaDEL | 3D | RDF25i | Radial distribution function - 025 / weighted by relative first ionization potential | RDF descriptor | 55.57 |
| PaDEL | 3D | RDF30i | Radial distribution function - 030 / weighted by relative first ionization potential | RDF descriptor | 11.14 |
| PaDEL | 3D | RDF35i | Radial distribution function - 035 / weighted by relative first ionization potential | RDF descriptor | 42.55 |
| PaDEL | 3D | RDF40i | Radial distribution function - 040 / weighted by relative first ionization potential | RDF descriptor | 30.12 |
| PaDEL | 3D | RDF45i | Radial distribution function - 045 / weighted by relative first ionization potential | RDF descriptor | 42.46 |
| PaDEL | 3D | RDF50i | Radial distribution function - 050 / weighted by relative first ionization potential | RDF descriptor | 48.7 |
| PaDEL | 3D | RDF55i | Radial distribution function - 055 / weighted by relative first ionization potential | RDF descriptor | 31.7 |
| PaDEL | 3D | RDF60i | Radial distribution function - 060 / weighted by relative first ionization potential | RDF descriptor | 48 |
| PaDEL | 3D | RDF65i | Radial distribution function - 065 / weighted by relative first ionization potential | RDF descriptor | 35.31 |
| PaDEL | 3D | RDF70i | Radial distribution function - 070 / weighted by relative first ionization potential | RDF descriptor | 35.35 |
| PaDEL | 3D | RDF75i | Radial distribution function - 075 / weighted by relative first ionization potential | RDF descriptor | 27.18 |
| PaDEL | 3D | RDF80i | Radial distribution function - 080 / weighted by relative first ionization potential | RDF descriptor | 22.73 |
| PaDEL | 3D | RDF85i | Radial distribution function - 085 / weighted by relative first ionization potential | RDF descriptor | 22.16 |
| PaDEL | 3D | RDF90i | Radial distribution function - 090 / weighted by relative first ionization potential | RDF descriptor | 18.61 |
| PaDEL | 3D | RDF95i | Radial distribution function - 095 / weighted by relative first ionization potential | RDF descriptor | 18.25 |
| PaDEL | 3D | RDF100i | Radial distribution function - 100 / weighted by relative first ionization potential | RDF descriptor | 15.52 |
| PaDEL | 3D | RDF105i | Radial distribution function - 105 / weighted by relative first ionization potential | RDF descriptor | 22.58 |
| PaDEL | 3D | RDF110i | Radial distribution function - 110 / weighted by relative first ionization potential | RDF descriptor | 14.04 |
| PaDEL | 3D | RDF115i | Radial distribution function - 115 / weighted by relative first ionization potential | RDF descriptor | 8.91 |
| PaDEL | 3D | RDF120i | Radial distribution function - 120 / weighted by relative first ionization potential | RDF descriptor | 10.36 |
| PaDEL | 3D | RDF125i | Radial distribution function - 125 / weighted by relative first ionization potential | RDF descriptor | 10.08 |
| PaDEL | 3D | RDF130i | Radial distribution function - 130 / weighted by relative first ionization potential | RDF descriptor | 6.2 |
| PaDEL | 3D | RDF135i | Radial distribution function - 135 / weighted by relative first ionization potential | RDF descriptor | 4.24 |
| PaDEL | 3D | RDF140i | Radial distribution function - 140 / weighted by relative first ionization potential | RDF descriptor | 2.71 |
| PaDEL | 3D | RDF145i | Radial distribution function - 145 / weighted by relative first ionization potential | RDF descriptor | 2.29 |
| PaDEL | 3D | RDF150i | Radial distribution function - 150 / weighted by relative first ionization potential | RDF descriptor | 2.48 |
| PaDEL | 3D | RDF155i | Radial distribution function - 155 / weighted by relative first ionization potential | RDF descriptor | 2.88 |
| PaDEL | 3D | RDF10s | Radial distribution function - 010 / weighted by relative I-state | RDF descriptor | 9.67 |
| PaDEL | 3D | RDF15s | Radial distribution function - 015 / weighted by relative I-state | RDF descriptor | 17.93 |
| PaDEL | 3D | RDF20s | Radial distribution function - 020 / weighted by relative I-state | RDF descriptor | 4.23 |
| PaDEL | 3D | RDF25s | Radial distribution function - 025 / weighted by relative I-state | RDF descriptor | 57.92 |
| PaDEL | 3D | RDF30s | Radial distribution function - 030 / weighted by relative I-state | RDF descriptor | 6.1 |
| PaDEL | 3D | RDF35s | Radial distribution function - 035 / weighted by relative I-state | RDF descriptor | 29.14 |
| PaDEL | 3D | RDF40s | Radial distribution function - 040 / weighted by relative I-state | RDF descriptor | 20.05 |
| PaDEL | 3D | RDF45s | Radial distribution function - 045 / weighted by relative I-state | RDF descriptor | 25.67 |
| PaDEL | 3D | RDF50s | Radial distribution function - 050 / weighted by relative I-state | RDF descriptor | 38.9 |
| PaDEL | 3D | RDF55s | Radial distribution function - 055 / weighted by relative I-state | RDF descriptor | 24.83 |
| PaDEL | 3D | RDF60s | Radial distribution function - 060 / weighted by relative I-state | RDF descriptor | 37.97 |
| PaDEL | 3D | RDF65s | Radial distribution function - 065 / weighted by relative I-state | RDF descriptor | 25.14 |
| PaDEL | 3D | RDF70s | Radial distribution function - 070 / weighted by relative I-state | RDF descriptor | 26.64 |
| PaDEL | 3D | RDF75s | Radial distribution function - 075 / weighted by relative I-state | RDF descriptor | 18.58 |
| PaDEL | 3D | RDF80s | Radial distribution function - 080 / weighted by relative I-state | RDF descriptor | 14.57 |
| PaDEL | 3D | RDF85s | Radial distribution function - 085 / weighted by relative I-state | RDF descriptor | 17.64 |
| PaDEL | 3D | RDF90s | Radial distribution function - 090 / weighted by relative I-state | RDF descriptor | 11.75 |
| PaDEL | 3D | RDF95s | Radial distribution function - 095 / weighted by relative I-state | RDF descriptor | 11.15 |
| PaDEL | 3D | RDF100s | Radial distribution function - 100 / weighted by relative I-state | RDF descriptor | 7.46 |
| PaDEL | 3D | RDF105s | Radial distribution function - 105 / weighted by relative I-state | RDF descriptor | 15.74 |
| PaDEL | 3D | RDF110s | Radial distribution function - 110 / weighted by relative I-state | RDF descriptor | 8.44 |
| PaDEL | 3D | RDF115s | Radial distribution function - 115 / weighted by relative I-state | RDF descriptor | 10.59 |
| PaDEL | 3D | RDF120s | Radial distribution function - 120 / weighted by relative I-state | RDF descriptor | 6.41 |
| PaDEL | 3D | RDF125s | Radial distribution function - 125 / weighted by relative I-state | RDF descriptor | 8.11 |
| PaDEL | 3D | RDF130s | Radial distribution function - 130 / weighted by relative I-state | RDF descriptor | 3.03 |
| PaDEL | 3D | RDF135s | Radial distribution function - 135 / weighted by relative I-state | RDF descriptor | 4.06 |
| PaDEL | 3D | RDF140s | Radial distribution function - 140 / weighted by relative I-state | RDF descriptor | 2.82 |
| PaDEL | 3D | RDF145s | Radial distribution function - 145 / weighted by relative I-state | RDF descriptor | 3.69 |
| PaDEL | 3D | RDF150s | Radial distribution function - 150 / weighted by relative I-state | RDF descriptor | 1.64 |
| PaDEL | 3D | RDF155s | Radial distribution function - 155 / weighted by relative I-state | RDF descriptor | 2.19 |
| PaDEL | 3D | L1u | 1st component size directional WHIM index / unweighted | PaDEL WHIM descriptor | 18.32 |
| PaDEL | 3D | L2u | 2nd component size directional WHIM index / unweighted | PaDEL WHIM descriptor | 4.18 |
| PaDEL | 3D | L3u | 3rd component size directional WHIM index / unweighted | PaDEL WHIM descriptor | 1.35 |
| PaDEL | 3D | P1u | 1st component shape directional WHIM index / unweighted | PaDEL WHIM descriptor | 0.77 |
| PaDEL | 3D | P2u | 2nd component shape directional WHIM index / unweighted | PaDEL WHIM descriptor | 0.18 |
| PaDEL | 3D | E1u | 1st component accessibility directional WHIM index / unweighted | PaDEL WHIM descriptor | 0.5 |
| PaDEL | 3D | E2u | 2nd component accessibility directional WHIM index / unweighted | PaDEL WHIM descriptor | 0.45 |
| PaDEL | 3D | E3u | 3rd component accessibility directional WHIM index / unweighted | PaDEL WHIM descriptor | 0.39 |
| PaDEL | 3D | Tu | T total size index / unweighted | PaDEL WHIM descriptor | 23.85 |
| PaDEL | 3D | Au | A total size index / unweighted | PaDEL WHIM descriptor | 106.98 |
| PaDEL | 3D | Vu | V total size index / unweighted | PaDEL WHIM descriptor | 234.1 |
| PaDEL | 3D | Ku | K global shape index / unweighted | PaDEL WHIM descriptor | 0.65 |
| PaDEL | 3D | Du | D total accessibility index / unweighted | PaDEL WHIM descriptor | 1.35 |
| PaDEL | 3D | L1m | 1st component size directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 16.06 |
| PaDEL | 3D | L2m | 2nd component size directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 3.58 |
| PaDEL | 3D | L3m | 3rd component size directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 0.6 |
| PaDEL | 3D | P1m | 1st component shape directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 0.79 |
| PaDEL | 3D | P2m | 2nd component shape directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 0.18 |
| PaDEL | 3D | E1m | 1st component accessibility directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 0.38 |
| PaDEL | 3D | E2m | 2nd component accessibility directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 0.32 |
| PaDEL | 3D | E3m | 3rd component accessibility directional WHIM index / weighted by relative mass | PaDEL WHIM descriptor | 0.07 |
| PaDEL | 3D | Tm | T total size index / weighted by relative mass | PaDEL WHIM descriptor | 20.25 |
| PaDEL | 3D | Am | A total size index / weighted by relative mass | PaDEL WHIM descriptor | 69.37 |
| PaDEL | 3D | Vm | V total size index / weighted by relative mass | PaDEL WHIM descriptor | 124.35 |
| PaDEL | 3D | Km | K global shape index / weighted by relative mass | PaDEL WHIM descriptor | 0.69 |
| PaDEL | 3D | Dm | D total accessibility index / weighted by relative mass | PaDEL WHIM descriptor | 0.78 |
| PaDEL | 3D | L1v | 1st component size directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 16.71 |
| PaDEL | 3D | L2v | 2nd component size directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 3.56 |
| PaDEL | 3D | L3v | 3rd component size directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.88 |
| PaDEL | 3D | P1v | 1st component shape directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.79 |
| PaDEL | 3D | P2v | 2nd component shape directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.17 |
| PaDEL | 3D | E1v | 1st component accessibility directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.42 |
| PaDEL | 3D | E2v | 2nd component accessibility directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.32 |
| PaDEL | 3D | E3v | 3rd component accessibility directional WHIM index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.16 |
| PaDEL | 3D | Tv | T total size index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 21.15 |
| PaDEL | 3D | Av | A total size index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 77.27 |
| PaDEL | 3D | Vv | V total size index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 150.54 |
| PaDEL | 3D | Kv | K global shape index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.69 |
| PaDEL | 3D | Dv | D total accessibility index / weighted by relative van der Waals volumes | PaDEL WHIM descriptor | 0.9 |
| PaDEL | 3D | L1e | 1st component size directional WHIM index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 18.2 |
| PaDEL | 3D | L2e | 2nd component size directional WHIM index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 4.22 |
| PaDEL | 3D | L3e | 3rd component size directional WHIM index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 1.29 |
| PaDEL | 3D | P1e | 1st component shape directional WHIM index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 0.77 |
| PaDEL | 3D | P2e | 2nd component shape directional WHIM index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 0.18 |
| PaDEL | 3D | E1e | 1st component accessibility directional WHIM index / weighted by relative Sanderson electronegativit | PaDEL WHIM descriptor | 0.49 |
| PaDEL | 3D | E2e | 2nd component accessibility directional WHIM index / weighted by relative Sanderson electronegativit | PaDEL WHIM descriptor | 0.46 |
| PaDEL | 3D | E3e | 3rd component accessibility directional WHIM index / weighted by relative Sanderson electronegativit | PaDEL WHIM descriptor | 0.35 |
| PaDEL | 3D | Te | T total size index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 23.71 |
| PaDEL | 3D | Ae | A total size index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 105.75 |
| PaDEL | 3D | Ve | V total size index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 228.47 |
| PaDEL | 3D | Ke | K global shape index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 0.65 |
| PaDEL | 3D | De | D total accessibility index / weighted by relative Sanderson electronegativities | PaDEL WHIM descriptor | 1.31 |
| PaDEL | 3D | L1p | 1st component size directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 17.12 |
| PaDEL | 3D | L2p | 2nd component size directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 3.62 |
| PaDEL | 3D | L3p | 3rd component size directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 1.02 |
| PaDEL | 3D | P1p | 1st component shape directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 0.79 |
| PaDEL | 3D | P2p | 2nd component shape directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 0.17 |
| PaDEL | 3D | E1p | 1st component accessibility directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 0.44 |
| PaDEL | 3D | E2p | 2nd component accessibility directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 0.34 |
| PaDEL | 3D | E3p | 3rd component accessibility directional WHIM index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 0.23 |
| PaDEL | 3D | Tp | T total size index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 21.76 |
| PaDEL | 3D | Ap | A total size index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 83.18 |
| PaDEL | 3D | Vp | V total size index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 168.36 |
| PaDEL | 3D | Kp | K global shape index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 0.68 |
| PaDEL | 3D | Dp | D total accessibility index / weighted by relative polarizabilities | PaDEL WHIM descriptor | 1 |
| PaDEL | 3D | L1i | 1st component size directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 18.57 |
| PaDEL | 3D | L2i | 2nd component size directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 4.31 |
| PaDEL | 3D | L3i | 3rd component size directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 1.41 |
| PaDEL | 3D | P1i | 1st component shape directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 0.76 |
| PaDEL | 3D | P2i | 2nd component shape directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 0.18 |
| PaDEL | 3D | E1i | 1st component accessibility directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 0.52 |
| PaDEL | 3D | E2i | 2nd component accessibility directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 0.48 |
| PaDEL | 3D | E3i | 3rd component accessibility directional WHIM index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 0.43 |
| PaDEL | 3D | Ti | T total size index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 24.29 |
| PaDEL | 3D | Ai | A total size index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 112.29 |
| PaDEL | 3D | Vi | V total size index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 249.53 |
| PaDEL | 3D | Ki | K global shape index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 0.65 |
| PaDEL | 3D | Di | D total accessibility index / weighted by relative first ionization potential | PaDEL WHIM descriptor | 1.42 |
| PaDEL | 3D | L1s | 1st component size directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 18.31 |
| PaDEL | 3D | L2s | 2nd component size directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 4.26 |
| PaDEL | 3D | L3s | 3rd component size directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 1.3 |
| PaDEL | 3D | P1s | 1st component shape directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 0.77 |
| PaDEL | 3D | P2s | 2nd component shape directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 0.18 |
| PaDEL | 3D | E1s | 1st component accessibility directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 0.5 |
| PaDEL | 3D | E2s | 2nd component accessibility directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 0.47 |
| PaDEL | 3D | E3s | 3rd component accessibility directional WHIM index / weighted by relative I-state | PaDEL WHIM descriptor | 0.36 |
| PaDEL | 3D | Ts | T total size index / weighted by relative I-state | PaDEL WHIM descriptor | 23.86 |
| PaDEL | 3D | As | A total size index / weighted by relative I-state | PaDEL WHIM descriptor | 107.28 |
| PaDEL | 3D | Vs | V total size index / weighted by relative I-state | PaDEL WHIM descriptor | 232.51 |
| PaDEL | 3D | Ks | K global shape index / weighted by relative I-state | PaDEL WHIM descriptor | 0.65 |
| PaDEL | 3D | Ds | D total accessibility index / weighted by relative I-state | PaDEL WHIM descriptor | 1.33 |