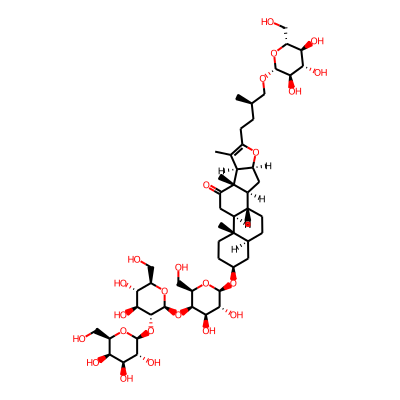
Summary
SMILES: OC[C@H]1O[C@@H](O[C@H]2CC[C@]3([C@H](C2)CC[C@@H]2[C@@H]3CC(=O)[C@]3([C@H]2C[C@H]2[C@@H]3C(=C(O2)CC[C@H](CO[C@@H]2O[C@H](CO)[C@H]([C@@H]([C@H]2O)O)O)C)C)C)C)[C@@H]([C@H]([C@H]1O[C@@H]1O[C@H](CO)[C@H]([C@@H]([C@H]1O[C@@H]1O[C@H](CO)[C@@H]([C@@H]([C@H]1O)O)O)O)O)O)OInChI: InChI=1S/C51H82O24/c1-19(18-67-46-41(64)37(60)34(57)28(14-52)70-46)5-8-26-20(2)33-27(69-26)12-25-23-7-6-21-11-22(9-10-50(21,3)24(23)13-32(56)51(25,33)4)68-47-43(66)40(63)44(31(17-55)73-47)74-49-45(39(62)36(59)30(16-54)72-49)75-48-42(65)38(61)35(58)29(15-53)71-48/h19,21-25,27-31,33-49,52-55,57-66H,5-18H2,1-4H3/t19-,21+,22+,23-,24+,25+,27+,28-,29-,30-,31-,33+,34-,35+,36-,37+,38+,39+,40-,41-,42-,43-,44+,45-,46-,47-,48+,49+,50+,51-/m1/s1InChIKey: TVRRDUXJKROMDX-CXLJPAHDSA-N
DeepSMILES: OC[C@H]O[C@@H]O[C@H]CC[C@][C@H]C6)CC[C@@H][C@@H]6CC=O)[C@][C@H]6C[C@H][C@@H]5C=CO5)CC[C@H]CO[C@@H]O[C@H]CO))[C@H][C@@H][C@H]6O))O))O)))))))C)))))C))))))C)))))))))C))))))[C@@H][C@H][C@H]6O[C@@H]O[C@H]CO))[C@H][C@@H][C@H]6O[C@@H]O[C@H]CO))[C@@H][C@@H][C@H]6O))O))O)))))))O))O)))))))O))O
Scaffold Graph/Node/Bond level: O=C1CC2C3CCC(OC4CCC(OC5OCCCC5OC5CCCCO5)CO4)CC3CCC2C2CC3OC(CCCCOC4CCCCO4)=CC3C12
Scaffold Graph/Node level: OC1CC2C3CCC(OC4CCC(OC5OCCCC5OC5CCCCO5)CO4)CC3CCC2C2CC3OC(CCCCOC4CCCCO4)CC3C12
Scaffold Graph level: CC1CC2C3CCC(CC4CCC(CC5CCCCC5CC5CCCCC5)CC4)CC3CCC2C2CC3CC(CCCCCC4CCCCC4)CC3C12
Functional groups: CC(C)=O; CC1=C(C)OCC1; CO; CO[C@@H](C)OC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Steroids and steroid derivatives
ClassyFire Subclass: Steroidal glycosides
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Steroids
NP Classifier Class: Furostane steroids
Synonymous chemical names:terrestrosin k
External chemical identifiers:CID:101707500
Chemical structure download