Summary
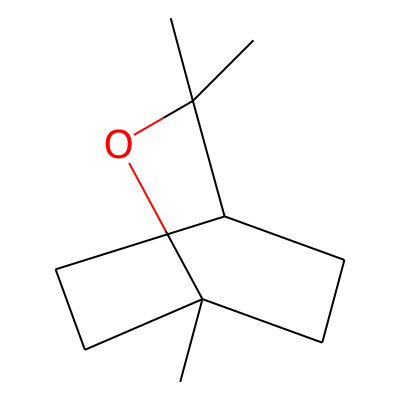
SMILES: CC12CCC(CC1)C(O2)(C)CInChI: InChI=1S/C10H18O/c1-9(2)8-4-6-10(3,11-9)7-5-8/h8H,4-7H2,1-3H3InChIKey: WEEGYLXZBRQIMU-UHFFFAOYSA-N
DeepSMILES: CCCCCCC6))CO6)C)C
Scaffold Graph/Node/Bond level: C1CC2CCC1CO2
Scaffold Graph/Node level: C1CC2CCC1CO2
Scaffold Graph level: C1CC2CCC1CC2
Functional groups: COC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Oxanes
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Monoterpenoids
NP Classifier Class: Menthane monoterpenoids|Monocyclic monoterpenoids
Synonymous chemical names:1 ,8-cineole, 1, 8- cineol, 1, 8-cineol, 1, 8-cineole, 1,8 - cineole, 1,8 cineol, 1,8 cineole, 1,8 cineole (eucalyptol), 1,8- cineole, 1,8-cinaole, 1,8-cineo1e, 1,8-cineoie, 1,8-cineol, 1,8-cineol/limonene, 1,8-cineole, 1,8-cineole (cineole), 1,8-cineole c, 1,8-cineole*, 1,8-cineole**, 1,8-cineole+, 1,8-cineole/limonene, 1,8-cineolebc, 1,8-cineole†, 1,8-cinéole, 1,8-ineole, 1,8_cineole, 1,8cineole, 1,8tineole, 1,8–cineole, 1-8 cineole-5, 1-8, cineole, 1-8,cineole, 1-8-cineole, 1-8_cineol, 1.8 cineole, 1.8-cineol, 1.8-cineole, 1.8-cineole*, 1_ 8-cineole, 1_8-cineole, cineol, cineol, 1,8, cineol, 1-8, cineol,1-8, cineol-1,8, cineole, cineole,1, 8-, cineole-1,8, cinéol-1,8, eucalyptol, eucalyptol (1,8-cineole), eucalyptol or 1,8-cineole
External chemical identifiers:CID:2758; ChEMBL:CHEMBL485259; ZINC:ZINC000000967566; SureChEMBL:SCHEMBL19622; MolPort-003-929-343
Chemical structure download