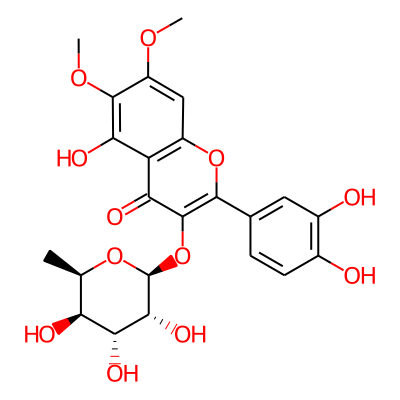
Summary
SMILES: COc1c(OC)cc2c(c1O)c(=O)c(c(o2)c1ccc(c(c1)O)O)O[C@@H]1O[C@H](C)[C@@H]([C@H]([C@H]1O)O)OInChI: InChI=1S/C23H24O12/c1-8-15(26)18(29)19(30)23(33-8)35-22-17(28)14-12(7-13(31-2)21(32-3)16(14)27)34-20(22)9-4-5-10(24)11(25)6-9/h4-8,15,18-19,23-27,29-30H,1-3H3/t8-,15+,18-,19-,23+/m1/s1InChIKey: NVZCGVLCUJLTSA-CTQDKRGWSA-N
DeepSMILES: COccOC))cccc6O))c=O)cco6)cccccc6)O))O))))))O[C@@H]O[C@H]C)[C@@H][C@H][C@H]6O))O))O
Scaffold Graph/Node/Bond level: O=c1c(OC2CCCCO2)c(-c2ccccc2)oc2ccccc12
Scaffold Graph/Node level: OC1C2CCCCC2OC(C2CCCCC2)C1OC1CCCCO1
Scaffold Graph level: CC1C2CCCCC2CC(C2CCCCC2)C1CC1CCCCC1
Functional groups: CO; c=O; cO; cOC; cO[C@@H](C)OC; coc
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Flavonoids
ClassyFire Subclass: Flavonoid glycosides
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Flavonoids
NP Classifier Class: Flavonols
Synonymous chemical names:eupatolin
External chemical identifiers:CID:22524218
Chemical structure download