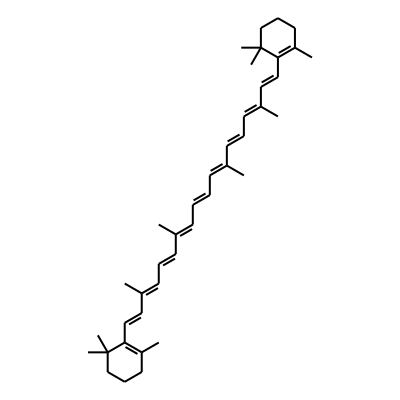
Summary
SMILES: C/C(=CC=CC=C(C=CC=C(C=CC1=C(C)CCCC1(C)C)/C)/C)/C=C/C=C(/C=C/C1=C(C)CCCC1(C)C)CInChI: InChI=1S/C40H56/c1-31(19-13-21-33(3)25-27-37-35(5)23-15-29-39(37,7)8)17-11-12-18-32(2)20-14-22-34(4)26-28-38-36(6)24-16-30-40(38,9)10/h11-14,17-22,25-28H,15-16,23-24,29-30H2,1-10H3/b12-11+,19-13+,20-14+,27-25+,28-26+,31-17+,32-18+,33-21+,34-22+InChIKey: OENHQHLEOONYIE-JLTXGRSLSA-N
DeepSMILES: C/C=CC=CC=CC=CC=CC=CC=CC)CCCC6C)C)))))))))/C)))))/C))))))/C=C/C=C/C=C/C=CC)CCCC6C)C)))))))))C
Scaffold Graph/Node/Bond level: C(=CC=CC=CC=CC=CC1=CCCCC1)C=CC=CC=CC=CC1=CCCCC1
Scaffold Graph/Node level: C(CCCCCCCCCC1CCCCC1)CCCCCCCCC1CCCCC1
Scaffold Graph level: C(CCCCCCCCCC1CCCCC1)CCCCCCCCC1CCCCC1
Functional groups: CC(C)=C(C)/C=C/C(C)=C/C=C/C(C)=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C(C)=C(C)C
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Tetraterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Carotenoids (C40)
NP Classifier Class: Carotenoids (C40, β-β)
Synonymous chemical names:(-) carotene, beta -carotene, beta carotene, beta-carotene, carotene, beta, neo beta carotene, pro-vitamin a, provitamin a, β-carotene
External chemical identifiers:CID:5280489; ChEMBL:CHEMBL1293; ChEBI:17579; ZINC:ZINC000006845076; FDASRS:01YAE03M7J; SureChEMBL:SCHEMBL6151; MolPort-001-785-959
Chemical structure download