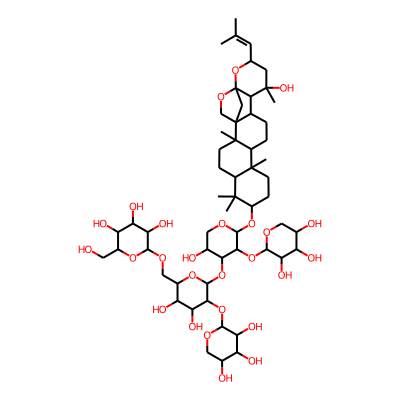
Summary
SMILES: OCC1OC(OCC2OC(OC3C(O)COC(C3OC3OCC(C(C3O)O)O)OC3CCC4(C(C3(C)C)CCC3(C4CCC4C53COC3(C5)C4C(C)(O)CC(O3)C=C(C)C)C)C)C(C(C2O)O)OC2OCC(C(C2O)O)O)C(C(C1O)O)OInChI: InChI=1S/C57H92O26/c1-23(2)14-24-15-55(7,71)46-25-8-9-32-53(5)12-11-33(52(3,4)31(53)10-13-54(32,6)56(25)21-57(46,83-24)76-22-56)79-50-45(82-49-41(69)35(63)27(60)18-73-49)43(28(61)19-74-50)80-51-44(81-48-40(68)34(62)26(59)17-72-48)39(67)37(65)30(78-51)20-75-47-42(70)38(66)36(64)29(16-58)77-47/h14,24-51,58-71H,8-13,15-22H2,1-7H3InChIKey: UOTFAVQIRLMJFF-UHFFFAOYSA-N
DeepSMILES: OCCOCOCCOCOCCO)COCC6OCOCCCC6O))O))O)))))))OCCCCCC6C)C))CCCC6CCCC6COCC5)C6CC)O)CCO6)C=CC)C)))))))))))))))C)))))C))))))))))))CCC6O))O))OCOCCCC6O))O))O))))))))))))CCC6O))O))O
Scaffold Graph/Node/Bond level: C1CCC(OCC2CCC(OC3CCCCO3)C(OC3CCOC(OC4CCC5C(CCC6C5CCC5C7CCCOC78CC65CO8)C4)C3OC3CCCCO3)O2)OC1
Scaffold Graph/Node level: C1CCC(OCC2CCC(OC3CCCCO3)C(OC3CCOC(OC4CCC5C(CCC6C5CCC5C7CCCOC78CC65CO8)C4)C3OC3CCCCO3)O2)OC1
Scaffold Graph level: C1CCC(CCC2CCC(CC3CCCCC3)C(CC3CCCC(CC4CCC5C(CCC6C5CCC5C7CCCCC78CCC65C8)C4)C3CC3CCCCC3)C2)CC1
Functional groups: CC=C(C)C; CO; COC(C)(C)OC; COC(C)OC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Triterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Triterpenoids
NP Classifier Class: Oleanane triterpenoids
Synonymous chemical names:hovenoside d
External chemical identifiers:CID:131751860
Chemical structure download