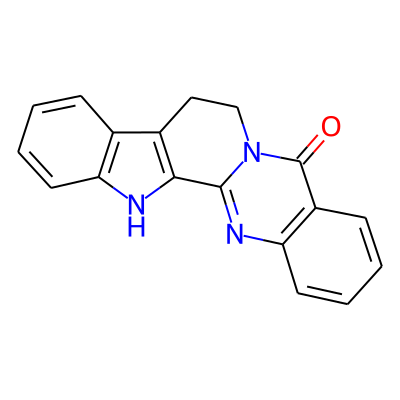
Summary
SMILES: O=c1c2ccccc2nc2-c3c(CCn12)c1c([nH]3)cccc1InChI: InChI=1S/C18H13N3O/c22-18-13-6-2-4-8-15(13)20-17-16-12(9-10-21(17)18)11-5-1-3-7-14(11)19-16/h1-8,19H,9-10H2InChIKey: ACVGWSKVRYFWRP-UHFFFAOYSA-N
DeepSMILES: O=ccccccc6nc-ccCCn%146)))cc[nH]5)cccc6
Scaffold Graph/Node/Bond level: O=c1c2ccccc2nc2n1CCc1c-2[nH]c2ccccc12
Scaffold Graph/Node level: OC1C2CCCCC2NC2C3NC4CCCCC4C3CCN12
Scaffold Graph level: CC1C2CCCCC2CC2C1CCC1C3CCCCC3CC12
Functional groups: c=O; c[nH]c; cn(c)C; cnc
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Indoles and derivatives
ClassyFire Subclass: Pyridoindoles
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tryptophan alkaloids|Anthranilic acid alkaloids
NP Classifier Class: Carboline alkaloids|Quinazoline alkaloids
Synonymous chemical names:rhetine, rutaccarpine, rutaecarpine
External chemical identifiers:CID:65752; ChEMBL:CHEMBL85139; ChEBI:8922; ZINC:ZINC000000898237; FDASRS:8XZV289PRY; SureChEMBL:SCHEMBL288507; MolPort-003-959-449
Chemical structure download