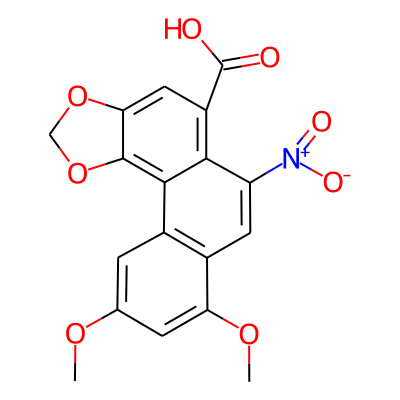
Summary
SMILES: COc1cc(OC)c2c(c1)c1c3OCOc3cc(c1c(c2)[N+](=O)[O-])C(=O)OInChI: InChI=1S/C18H13NO8/c1-24-8-3-10-9(13(4-8)25-2)5-12(19(22)23)15-11(18(20)21)6-14-17(16(10)15)27-7-26-14/h3-6H,7H2,1-2H3,(H,20,21)InChIKey: GYBINMVKWZEICQ-UHFFFAOYSA-N
DeepSMILES: COcccOC))ccc6)ccOCOc5ccc9cc%13)[N+]=O)[O-]))))C=O)O
Scaffold Graph/Node/Bond level: c1ccc2c(c1)ccc1ccc3c(c12)OCO3
Scaffold Graph/Node level: C1CCC2C(C1)CCC1CCC3OCOC3C12
Scaffold Graph level: C1CCC2C(C1)CCC1CCC3CCCC3C12
Functional groups: c1cOCO1; cC(=O)O; cOC; c[N+](=O)[O-]
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: BenzenoidsClassyFire Class: Phenanthrenes and derivatives
ClassyFire Subclass: Aristolochic acids and derivatives
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tyrosine alkaloids
NP Classifier Class: Aporphine alkaloids
Synonymous chemical names:aristolochic acid iv, aristolochic acid iv methylether
External chemical identifiers:CID:167493; ChEMBL:CHEMBL465004
Chemical structure download