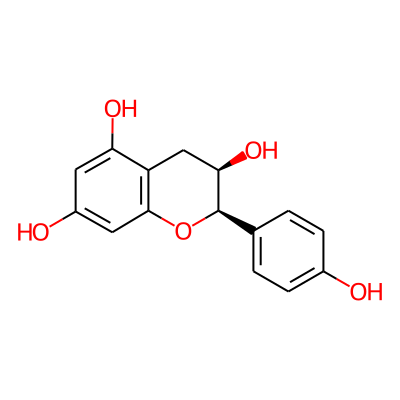
Summary
SMILES: Oc1ccc(cc1)[C@H]1Oc2cc(O)cc(c2C[C@H]1O)OInChI: InChI=1S/C15H14O5/c16-9-3-1-8(2-4-9)15-13(19)7-11-12(18)5-10(17)6-14(11)20-15/h1-6,13,15-19H,7H2/t13-,15-/m1/s1InChIKey: RSYUFYQTACJFML-UKRRQHHQSA-N
DeepSMILES: Occcccc6))[C@H]OcccO)ccc6C[C@H]%10O))))O
Scaffold Graph/Node/Bond level: c1ccc(C2CCc3ccccc3O2)cc1
Scaffold Graph/Node level: C1CCC(C2CCC3CCCCC3O2)CC1
Scaffold Graph level: C1CCC(C2CCC3CCCCC3C2)CC1
Functional groups: CO; cO; cOC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Flavonoids
ClassyFire Subclass: Flavans
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Flavonoids
NP Classifier Class: Flavan-3-ols
Synonymous chemical names:(-)-epiafzelechin, (-)-epiafzelichin, (-)epiafzelechin, afzelechin, epi (-), epiafazelchin, epiafzelechin, epiafzelechin,, epiafzelechin,(_)-
External chemical identifiers:CID:443639; ChEMBL:CHEMBL159303; ChEBI:31028; ZINC:ZINC000001721694; SureChEMBL:SCHEMBL557061; MolPort-003-665-792
Chemical structure download