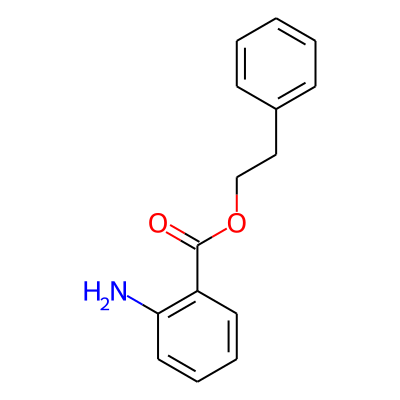
Summary
SMILES: O=C(c1ccccc1N)OCCc1ccccc1InChI: InChI=1S/C15H15NO2/c16-14-9-5-4-8-13(14)15(17)18-11-10-12-6-2-1-3-7-12/h1-9H,10-11,16H2InChIKey: PXWNBAGCFUDYBE-UHFFFAOYSA-N
DeepSMILES: O=Ccccccc6N)))))))OCCcccccc6
Scaffold Graph/Node/Bond level: O=C(OCCc1ccccc1)c1ccccc1
Scaffold Graph/Node level: OC(OCCC1CCCCC1)C1CCCCC1
Scaffold Graph level: CC(CCCC1CCCCC1)C1CCCCC1
Functional groups: cC(=O)OC; cN
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: BenzenoidsClassyFire Class: Benzene and substituted derivatives
ClassyFire Subclass: Benzoic acids and derivatives
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Anthranilic acid alkaloids
NP Classifier Class: Anthranillic acid derivatives
Synonymous chemical names:2-phenyl ethyl anthranilate, 2-phenylethyl anthranilate, phenylethyl anthranilate, phenylethylanthranilate
External chemical identifiers:CID:8615; ChEMBL:CHEMBL1488668; ZINC:ZINC000001693901; FDASRS:8HBK71OD81; SureChEMBL:SCHEMBL446803
Chemical structure download