Summary
IMPPAT Phytochemical identifier: IMPHY000220
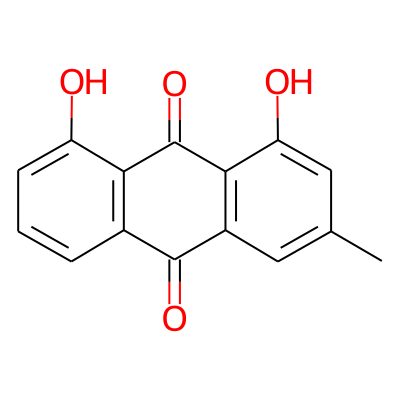
Phytochemical name: Chrysophanol
Synonymous chemical names:1,8-dihydroxy-3 methylanthraquinone, 1,8-dihydroxy-3-methylanthraquinone, archinin(chrysophanic acid), chrysophanic acid, chrysophanic-acid, chrysophanol
External chemical identifiers:CID:10208, ChEMBL:CHEMBL41092, ChEBI:3687, ZINC:ZINC000003861630, FDASRS:N1ST8V8RR2, SureChEMBL:SCHEMBL308131, MolPort-000-165-353
Chemical structure information
SMILES:
Cc1cc(O)c2c(c1)C(=O)c1c(C2=O)c(O)ccc1InChI:
InChI=1S/C15H10O4/c1-7-5-9-13(11(17)6-7)15(19)12-8(14(9)18)3-2-4-10(12)16/h2-6,16-17H,1H3InChIKey:
LQGUBLBATBMXHT-UHFFFAOYSA-NDeepSMILES:
CcccO)ccc6)C=O)ccC6=O))cO)ccc6Functional groups:
cC(c)=O, cO
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C1c2ccccc2C(=O)c2ccccc21Scaffold Graph/Node level:
OC1C2CCCCC2C(O)C2CCCCC12Scaffold Graph level:
CC1C2CCCCC2C(C)C2CCCCC12
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: BenzenoidsClassyFire Class: Anthracenes
ClassyFire Subclass: Anthraquinones
NP Classifier Biosynthetic pathway: Polyketides
NP Classifier Superclass: Polycyclic aromatic polyketides
NP Classifier Class: Anthraquinones and anthrones
NP-Likeness score: 1.035
Chemical structure download