Summary
IMPPAT Phytochemical identifier: IMPHY000274
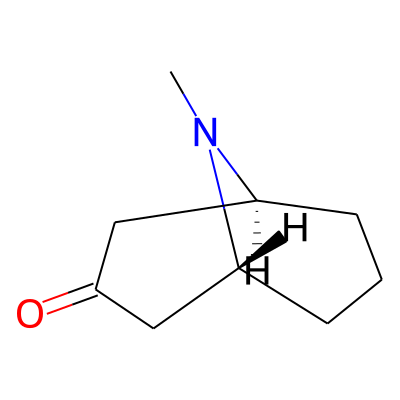
Phytochemical name: 9-Azabicyclo(3.3.1)nonan-3-one, 9-methyl-
Synonymous chemical names:9-methyl-9-azabicyclo (3.3.1)nonan-3-one (granatan-3-one), granatan-3-one, pseudo pelletierine, pseudopelletierine, pseudopelletierine (n-methylgranatonine), pseudopelletierine(n-methylgranatonine)
External chemical identifiers:CID:6602484, ChEMBL:CHEMBL1329064, ChEBI:8607, ZINC:ZINC000004098911, FDASRS:USN3FV3Z9X, SureChEMBL:SCHEMBL13908059, MolPort-003-925-669
Chemical structure information
SMILES:
O=C1C[C@@H]2CCC[C@H](C1)N2CInChI:
InChI=1S/C9H15NO/c1-10-7-3-2-4-8(10)6-9(11)5-7/h7-8H,2-6H2,1H3/t7-,8+InChIKey:
RHWSKVCZXBAWLZ-OCAPTIKFSA-NDeepSMILES:
O=CC[C@@H]CCC[C@H]C8)N6CFunctional groups:
CC(C)=O, CN(C)C
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C1CC2CCCC(C1)N2Scaffold Graph/Node level:
OC1CC2CCCC(C1)N2Scaffold Graph level:
CC1CC2CCCC(C1)C2
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Piperidines
ClassyFire Subclass: Piperidinones
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Lysine alkaloids
NP Classifier Class: Piperidine alkaloids
NP-Likeness score: 0.669
Chemical structure download