Summary
IMPPAT Phytochemical identifier: IMPHY001348
Phytochemical name: Morphine N-oxide
Synonymous chemical names:morphine n-oxide, morphine-n-oxide
External chemical identifiers:CID:5362459, ChEBI:7002, ZINC:ZINC000004102209, FDASRS:9E77NL2Y9I, SureChEMBL:SCHEMBL619646
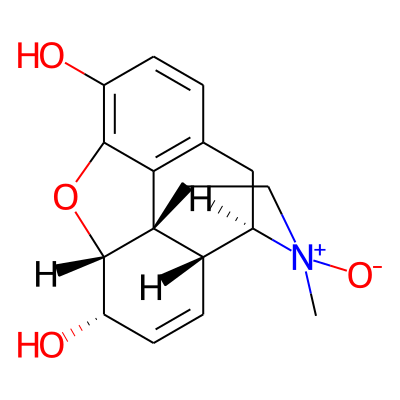
Chemical structure information
SMILES:
O[C@H]1C=C[C@@H]2[C@@]34[C@H]1Oc1c4c(C[C@H]2[N+](CC3)([O-])C)ccc1OInChI:
InChI=1S/C17H19NO4/c1-18(21)7-6-17-10-3-5-13(20)16(17)22-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-,18?/m0/s1InChIKey:
AMAPEXTUMXQULJ-APQDOHRLSA-NDeepSMILES:
O[C@H]C=C[C@@H][C@][C@H]6Occ5cC[C@H]9[N+]CC%11))[O-])C))))ccc6OFunctional groups:
CC=CC, CO, C[N+](C)(C)[O-], cO, cOC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C1=CC2C3Cc4cccc5c4C2(CC[NH2+]3)C(C1)O5Scaffold Graph/Node level:
C1CC2CC3NCCC45C(CCCC34)OC(C1)C25Scaffold Graph level:
C1CC2CC3CCCC45C(CCCC34)CC(C1)C25
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Alkaloids and derivativesClassyFire Class: Morphinans
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tyrosine alkaloids
NP Classifier Class: Morphinan alkaloids, Isoquinoline alkaloids
NP-Likeness score: 2.643
Chemical structure download