Summary
IMPPAT Phytochemical identifier: IMPHY001605
Phytochemical name: Neferine
Synonymous chemical names:neferine
External chemical identifiers:CID:159654, ChEMBL:CHEMBL455560, ChEBI:176257, ZINC:ZINC000006017976, SureChEMBL:SCHEMBL12807595, MolPort-003-804-322
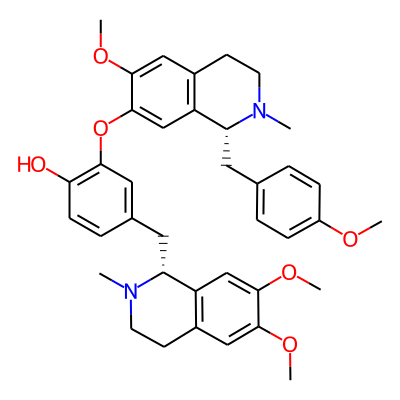
Chemical structure information
SMILES:
COc1ccc(cc1)C[C@H]1N(C)CCc2c1cc(Oc1cc(ccc1O)C[C@H]1N(C)CCc3c1cc(OC)c(c3)OC)c(c2)OCInChI:
InChI=1S/C38H44N2O6/c1-39-15-14-27-21-36(44-5)38(23-30(27)31(39)17-24-7-10-28(42-3)11-8-24)46-34-19-25(9-12-33(34)41)18-32-29-22-37(45-6)35(43-4)20-26(29)13-16-40(32)2/h7-12,19-23,31-32,41H,13-18H2,1-6H3/t31-,32-/m1/s1InChIKey:
MIBATSHDJRIUJK-ROJLCIKYSA-NDeepSMILES:
COcccccc6))C[C@H]NC)CCcc6ccOcccccc6O))))C[C@H]NC)CCcc6ccOC))cc6)OC))))))))))))))))cc6)OCFunctional groups:
CN(C)C, cO, cOC, cOc
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccc(CC2NCCc3ccc(Oc4cccc(CC5NCCc6ccccc65)c4)cc32)cc1Scaffold Graph/Node level:
C1CCC(CC2NCCC3CCC(OC4CCCC(CC5NCCC6CCCCC65)C4)CC32)CC1Scaffold Graph level:
C1CCC(CC2CCCC3CCC(CC4CCCC(CC5CCCC6CCCCC65)C4)CC32)CC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Isoquinolines and derivatives
ClassyFire Subclass: Benzylisoquinolines
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tyrosine alkaloids
NP Classifier Class: Isoquinoline alkaloids, Tetrahydroisoquinoline alkaloids
NP-Likeness score: 0.547
Chemical structure download