Summary
IMPPAT Phytochemical identifier: IMPHY001701
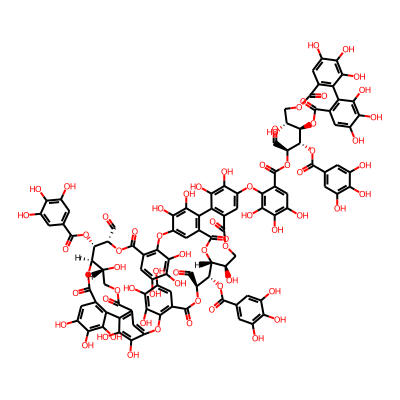
Phytochemical name: Oenothein A
Synonymous chemical names:oenothein a
External chemical identifiers:CID:16130421
Chemical structure information
SMILES:
O=C[C@@H]1OC(=O)c2cc(O)c(c(c2Oc2cc3C(=O)OC[C@@H](O)[C@@H](OC(=O)c4c(-c3c(c2O)O)c(O)c(c(c4)O)O)[C@@H]([C@@H](OC(=O)c2c(Oc3cc4C(=O)O[C@@H]([C@@H]1OC(=O)c1cc(O)c(c(c1)O)O)[C@H](O)COC(=O)c1c(-c4c(c3O)O)c(O)c(c(c1)Oc1c(cc(c(c1O)O)O)C(=O)O[C@H]([C@H]([C@@H]1OC(=O)c3cc(O)c(c(c3-c3c(C(=O)OC[C@H]1O)cc(c(c3O)O)O)O)O)OC(=O)c1cc(O)c(c(c1)O)O)C=O)O)c(O)c(c(c2)O)O)C=O)OC(=O)c1cc(O)c(c(c1)O)O)O)OInChI:
InChI=1S/C102H72O66/c103-16-52(88(166-91(142)22-1-34(106)61(121)35(107)2-22)85-46(118)19-154-94(145)25-7-40(112)64(124)73(133)55(25)56-26(97(148)163-85)8-41(113)65(125)74(56)134)160-100(151)32-11-44(116)68(128)80(140)83(32)158-50-14-29-59(77(137)71(50)131)60-30-15-51(72(132)78(60)138)159-84-33(12-45(117)69(129)81(84)141)102(153)161-53(17-104)89(167-92(143)23-3-36(108)62(122)37(109)4-23)86-47(119)20-155-95(146)28-13-49(70(130)76(136)58(28)57-27(98(149)164-86)9-42(114)66(126)75(57)135)157-82-31(10-43(115)67(127)79(82)139)101(152)162-54(18-105)90(87(165-99(30)150)48(120)21-156-96(29)147)168-93(144)24-5-38(110)63(123)39(111)6-24/h1-18,46-48,52-54,85-90,106-141H,19-21H2/t46-,47-,48-,52+,53+,54+,85-,86-,87-,88-,89-,90-/m1/s1InChIKey:
BJKKYEKHHYWTQZ-BHGGTIJZSA-NDeepSMILES:
O=C[C@@H]OC=O)cccO)ccc6OcccC=O)OC[C@@H]O)[C@@H]OC=O)cc-c%11cc%15O))O)))cO)ccc6)O))O)))))))[C@@H][C@@H]OC=O)ccOcccC=O)O[C@@H][C@@H]%32OC=O)cccO)ccc6)O))O))))))))[C@H]O)COC=O)cc-c%11cc%15O))O)))cO)ccc6)Occcccc6O))O))O)))C=O)O[C@H][C@H][C@@H]OC=O)cccO)ccc6-ccC=O)OC[C@H]%15O)))))cccc6O))O))O))))))O))O))))))))OC=O)cccO)ccc6)O))O))))))))C=O)))))))))O))))))))))))))))cO)ccc6)O))O)))))))C=O)))OC=O)cccO)ccc6)O))O))))))))))))))))))O))OFunctional groups:
CC=O, CO, cC(=O)OC, cO, cOc
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C(OC(COC(=O)c1ccccc1Oc1ccc2c(c1)C(=O)OCCC1OC(=O)c3cc(ccc3-2)Oc2ccccc2C(=O)OCC(OC(=O)c2ccccc2)C2CCOC(=O)c3cc(ccc3-c3ccccc3C(=O)O2)Oc2ccccc2C(=O)OCC1OC(=O)c1ccccc1)C1CCOC(=O)c2ccccc2-c2ccccc2C(=O)O1)c1ccccc1Scaffold Graph/Node level:
OC(OC(COC(O)C1CCCCC1OC1CCC2C(C1)C(O)OCCC1OC(O)C3CC(CCC32)OC2CCCCC2C(O)OCC(OC(O)C2CCCCC2)C2CCOC(O)C3CC(CCC3C3CCCCC3C(O)O2)OC2CCCCC2C(O)OCC1OC(O)C1CCCCC1)C1CCOC(O)C2CCCCC2C2CCCCC2C(O)O1)C1CCCCC1Scaffold Graph level:
CC(CC(CCC(C)C1CCCCC1CC1CCC2C(C1)C(C)CCCC1CC(C)C3CC(CCC32)CC2CCCCC2C(C)CCC(CC(C)C2CCCCC2)C2CCCC(C)C3CC(CCC3C3CCCCC3C(C)C2)CC2CCCCC2C(C)CCC1CC(C)C1CCCCC1)C1CCCC(C)C2CCCCC2C2CCCCC2C(C)C1)C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Tannins
ClassyFire Subclass: Hydrolyzable tannins
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Phenolic acids (C6-C1)
NP Classifier Class: Gallotannins
NP-Likeness score: 0.683
Chemical structure download