IMPPAT Phytochemical information:
Chamaecydin
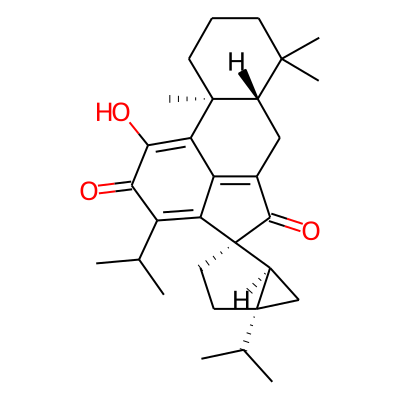
Summary
IMPPAT Phytochemical identifier: IMPHY002173
Phytochemical name: Chamaecydin
Synonymous chemical names:chamaecydin
External chemical identifiers:CID:101637219, ZINC:ZINC000169641498
Chemical structure information
SMILES:
CC(C1=C2C3=C(C(=O)[C@@]42CC[C@]2([C@@H]4C2)C(C)C)C[C@@H]2[C@](C3=C(C1=O)O)(C)CCCC2(C)C)CInChI:
InChI=1S/C30H40O3/c1-15(2)20-22-21-17(26(33)30(22)12-11-29(16(3)4)14-19(29)30)13-18-27(5,6)9-8-10-28(18,7)23(21)25(32)24(20)31/h15-16,18-19,32H,8-14H2,1-7H3/t18-,19-,28-,29+,30-/m0/s1InChIKey:
CTGGVCKBMLNHNX-WLHXYQFRSA-NDeepSMILES:
CCC=CC=CC=O)[C@]5CC[C@][C@@H]5C3))CC)C)))))))C[C@@H][C@]C6=CC%10=O))O)))C)CCCC6C)C))))))))))))CFunctional groups:
CC1=C2CC(=O)C(C)=C2C(C)=C(O)C1=O
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C1C=C2C3=C(CC4CCCCC24)C(=O)C2(CCC4CC42)C3=C1Scaffold Graph/Node level:
OC1CC2C3CCCCC3CC3C2C(C1)C1(CCC2CC21)C3OScaffold Graph level:
CC1CC2C3CCCCC3CC3C2C(C1)C1(CCC2CC21)C3C
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Diterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Diterpenoids
NP Classifier Class: Abietane diterpenoids
NP-Likeness score: 2.176
Chemical structure download
