Summary
IMPPAT Phytochemical identifier: IMPHY003392
Phytochemical name: Butylated hydroxytoluene
Synonymous chemical names:2, 6-di-tert-butyl- p-cresol, 2,6-di-tert-butyl-4-methylphenol, 2-6-ditert-butyl-4-methyl-phenol, buthylhydroxy tholuene, butylated hydroxytoluene, butylated hydroxytoluene, topanol, vianol, butylated hydroxytoulene, butylatedhydroxytoluene, di-ter-butyl p-cresol, di-ter-butyl-p-cresol, ionol
External chemical identifiers:CID:31404, ChEMBL:CHEMBL146, ChEBI:34247, ZINC:ZINC000001481993, FDASRS:1P9D0Z171K, SureChEMBL:SCHEMBL3950, MolPort-000-872-015
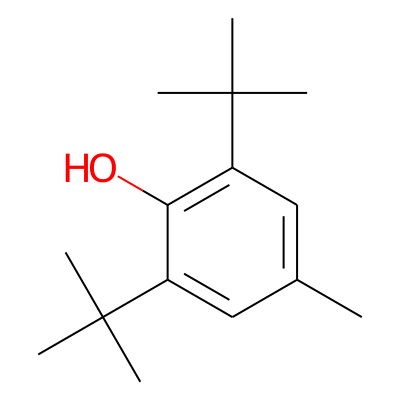
Chemical structure information
SMILES:
Cc1cc(c(c(c1)C(C)(C)C)O)C(C)(C)CInChI:
InChI=1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3InChIKey:
NLZUEZXRPGMBCV-UHFFFAOYSA-NDeepSMILES:
Ccccccc6)CC)C)C)))O))CC)C)CFunctional groups:
cO
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccccc1Scaffold Graph/Node level:
C1CCCCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: BenzenoidsClassyFire Class: Benzene and substituted derivatives
ClassyFire Subclass: Phenylpropanes
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP-Likeness score: 0.071
Chemical structure download