Summary
IMPPAT Phytochemical identifier: IMPHY003523
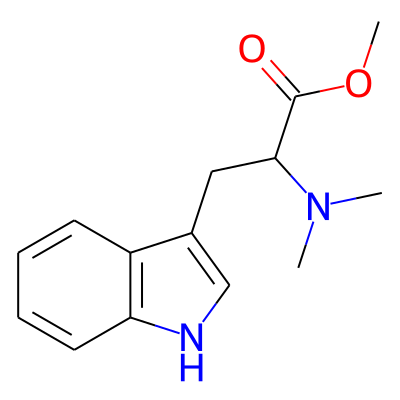
Phytochemical name: Methyl 2-(dimethylamino)-3-(1H-indol-3-yl)propanoate
Synonymous chemical names:n,n-dimethyl tryptophan methyl ester, n,n-dimethyltryptophan methyl ester
External chemical identifiers:CID:289078, ChEMBL:CHEMBL273276, SureChEMBL:SCHEMBL10867367
Chemical structure information
SMILES:
COC(=O)C(N(C)C)Cc1c[nH]c2c1cccc2InChI:
InChI=1S/C14H18N2O2/c1-16(2)13(14(17)18-3)8-10-9-15-12-7-5-4-6-11(10)12/h4-7,9,13,15H,8H2,1-3H3InChIKey:
QFHMLRWKLHONAO-UHFFFAOYSA-NDeepSMILES:
COC=O)CNC)C))Ccc[nH]cc5cccc6Functional groups:
CN(C)C, COC(C)=O, c[nH]c
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccc2[nH]ccc2c1Scaffold Graph/Node level:
C1CCC2NCCC2C1Scaffold Graph level:
C1CCC2CCCC2C1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organic acids and derivativesClassyFire Class: Carboxylic acids and derivatives
ClassyFire Subclass: Amino acids, peptides, and analogues
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tryptophan alkaloids
NP Classifier Class: Simple indole alkaloids
NP-Likeness score: 0.017
Chemical structure download