Summary
IMPPAT Phytochemical identifier: IMPHY003636
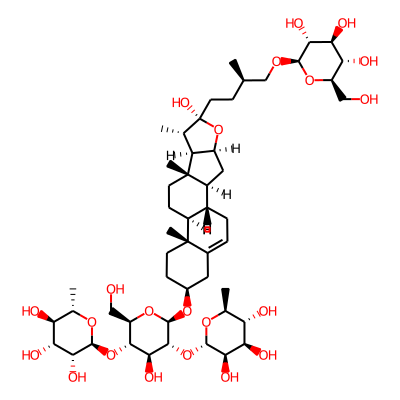
Phytochemical name: Protodioscin
Synonymous chemical names:dioscin, proto, protodioscin
External chemical identifiers:CID:441891, ChEBI:8588, FDASRS:D0LC3PH24P, SureChEMBL:SCHEMBL786390, MolPort-006-069-135
Chemical structure information
SMILES:
OC[C@H]1O[C@@H](O[C@H]2CC[C@]3(C(=CC[C@@H]4[C@@H]3CC[C@]3([C@H]4C[C@H]4[C@@H]3[C@H](C)[C@@](O4)(O)CC[C@H](CO[C@@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O)C)C)C2)C)[C@@H]([C@H]([C@@H]1O[C@@H]1O[C@@H](C)[C@@H]([C@H]([C@H]1O)O)O)O)O[C@@H]1O[C@@H](C)[C@@H]([C@H]([C@H]1O)O)OInChI:
InChI=1S/C51H84O22/c1-20(19-65-45-39(60)38(59)35(56)30(17-52)69-45)9-14-51(64)21(2)32-29(73-51)16-28-26-8-7-24-15-25(10-12-49(24,5)27(26)11-13-50(28,32)6)68-48-44(72-47-41(62)37(58)34(55)23(4)67-47)42(63)43(31(18-53)70-48)71-46-40(61)36(57)33(54)22(3)66-46/h7,20-23,25-48,52-64H,8-19H2,1-6H3/t20-,21+,22+,23+,25+,26-,27+,28+,29+,30-,31-,32+,33+,34+,35-,36-,37-,38+,39-,40-,41-,42+,43-,44-,45-,46+,47+,48-,49+,50+,51-/m1/s1InChIKey:
LVTJOONKWUXEFR-UEZXSUPNSA-NDeepSMILES:
OC[C@H]O[C@@H]O[C@H]CC[C@]C=CC[C@@H][C@@H]6CC[C@][C@H]6C[C@H][C@@H]5[C@H]C)[C@@]O5)O)CC[C@H]CO[C@@H]O[C@H]CO))[C@H][C@@H][C@H]6O))O))O)))))))C))))))))))C))))))))C6))C))))))[C@@H][C@H][C@@H]6O[C@@H]O[C@@H]C)[C@@H][C@H][C@H]6O))O))O)))))))O))O[C@@H]O[C@@H]C)[C@@H][C@H][C@H]6O))O))OFunctional groups:
CC=C(C)C, CO, CO[C@@H](C)OC, CO[C@H](C)OC, C[C@@](C)(O)OC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C1=C2CC(OC3OCC(OC4CCCCO4)CC3OC3CCCCO3)CCC2C2CCC3C4CC(CCCCOC5CCCCO5)OC4CC3C2C1Scaffold Graph/Node level:
C(CCC1CC2C(CC3C2CCC2C4CCC(OC5OCC(OC6CCCCO6)CC5OC5CCCCO5)CC4CCC23)O1)COC1CCCCO1Scaffold Graph level:
C1CCC(CCCCCC2CC3CC4C(CCC5C6CCC(CC7CCC(CC8CCCCC8)CC7CC7CCCCC7)CC6CCC54)C3C2)CC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Steroids and steroid derivatives
ClassyFire Subclass: Steroidal glycosides
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Steroids
NP Classifier Class: Furostane steroids
NP-Likeness score: 2.11
Chemical structure download