Summary
IMPPAT Phytochemical identifier: IMPHY005758
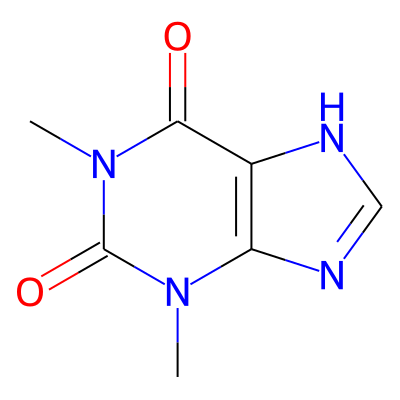
Phytochemical name: Theophylline
Synonymous chemical names:theophylline
External chemical identifiers:CID:2153, ChEMBL:CHEMBL190, ChEBI:28177, ZINC:ZINC000018043251, FDASRS:0I55128JYK, SureChEMBL:SCHEMBL4915, MolPort-001-737-342
Chemical structure information
SMILES:
Cn1c(=O)n(C)c2c(c1=O)[nH]cn2InChI:
InChI=1S/C7H8N4O2/c1-10-5-4(8-3-9-5)6(12)11(2)7(10)13/h3H,1-2H3,(H,8,9)InChIKey:
ZFXYFBGIUFBOJW-UHFFFAOYSA-NDeepSMILES:
Cnc=O)nC)ccc6=O))[nH]cn5Functional groups:
c=O, c[nH]c, cn(c)C, cnc
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=c1[nH]c(=O)c2[nH]cnc2[nH]1Scaffold Graph/Node level:
OC1NC(O)C2NCNC2N1Scaffold Graph level:
CC1CC(C)C2CCCC2C1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Imidazopyrimidines
ClassyFire Subclass: Purines and purine derivatives
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Pseudoalkaloids
NP Classifier Class: Purine alkaloids
NP-Likeness score: -0.75
Chemical structure download