Summary
IMPPAT Phytochemical identifier: IMPHY006145
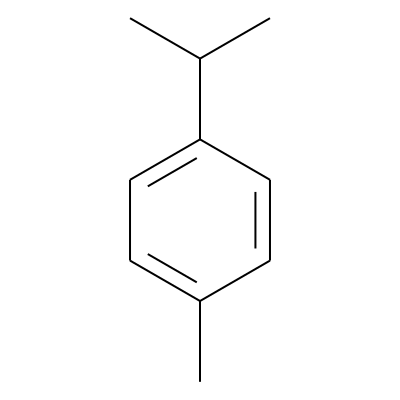
Phytochemical name: p-Cymene
Synonymous chemical names:*p-cymene, 1-methoxy-4-isopropyl-benzene, cymene, cymene, p-, cymene,p-, cymol, cymol,p-, m,o or p-cymene, p -cymene, p- cymene, p-cimene, p-cymcne, p-cymeme, p-cymene, p-cymene†, p-cymol, p-cyrnene, para-cymene, p‐cymene
External chemical identifiers:CID:7463, ChEMBL:CHEMBL442915, ChEBI:28768, ZINC:ZINC000000968246, FDASRS:1G1C8T1N7Q, SureChEMBL:SCHEMBL1143, MolPort-003-929-568
Chemical structure information
SMILES:
Cc1ccc(cc1)C(C)CInChI:
InChI=1S/C10H14/c1-8(2)10-6-4-9(3)5-7-10/h4-8H,1-3H3InChIKey:
HFPZCAJZSCWRBC-UHFFFAOYSA-NDeepSMILES:
Ccccccc6))CC)C
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccccc1Scaffold Graph/Node level:
C1CCCCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Monoterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Monoterpenoids
NP Classifier Class: Menthane monoterpenoids
NP-Likeness score: -0.717
Chemical structure download