Summary
IMPPAT Phytochemical identifier: IMPHY007331
Phytochemical name: 6-Methyl-5-hepten-2-one
Synonymous chemical names:2-methyl-hept-2-en-6-one, 6- methyl-5-hepten-2-one, 6-methy-5-hepten-2-one, 6-methy1-5-hepten-2-one, 6-methyk-hepten-2-one, 6-methyl 5 hepten-2-one, 6-methyl hept-5-en-2-one, 6-methyl hept-5-ene-2-one, 6-methyl-5 hepten-2-one, 6-methyl-5-hepten-2-one, 6-methyl-5-hepten-2-one*, 6-methyl-5-hepten-2-one,, 6-methyl-5-hepten-2one, 6-methyl-hept-5-en-2-one, 6-methylhept-5-en-2-0ne, 6-methylhept-5-en-2-one, 6-methyl–5-hepten-2-one, methyl hcptenone, methyl heptenone, methylheptenone, sulcatone
External chemical identifiers:CID:9862, ChEMBL:CHEMBL46340, ChEBI:16310, ZINC:ZINC000000896810, FDASRS:448353S93V, SureChEMBL:SCHEMBL157735, MolPort-001-788-424
Chemical structure information
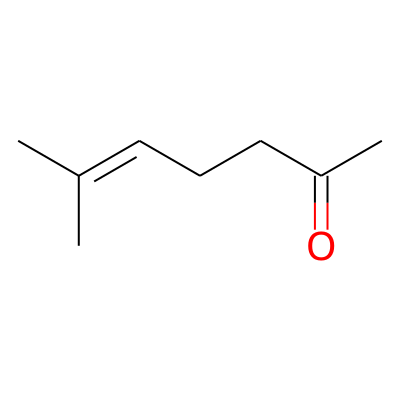
SMILES:
CC(=O)CCC=C(C)CInChI:
InChI=1S/C8H14O/c1-7(2)5-4-6-8(3)9/h5H,4,6H2,1-3H3InChIKey:
UHEPJGULSIKKTP-UHFFFAOYSA-NDeepSMILES:
CC=O)CCC=CC)CFunctional groups:
CC(C)=O, CC=C(C)C
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organic oxygen compoundsClassyFire Class: Organooxygen compounds
ClassyFire Subclass: Carbonyl compounds
NP Classifier Biosynthetic pathway: Fatty acids, Terpenoids
NP Classifier Superclass: Apocarotenoids, Fatty acyls, Monoterpenoids
NP Classifier Class: Acyclic monoterpenoids, Apocarotenoids (Ψ-), Oxygenated hydrocarbons
NP-Likeness score: 2.124
Chemical structure download