Summary
IMPPAT Phytochemical identifier: IMPHY007838
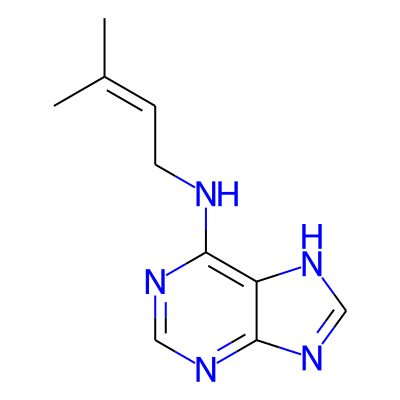
Phytochemical name: N-(3-Methylbut-2-EN-1-YL)-9H-purin-6-amine
Synonymous chemical names:isopentenyl-adenine, isopentenyladenine, n6-isopentenyladenine
External chemical identifiers:CID:92180, ChEMBL:CHEMBL476189, ChEBI:17660, ZINC:ZINC000000032351, FDASRS:V500BB256Z, SureChEMBL:SCHEMBL459276, MolPort-044-183-408
Chemical structure information
SMILES:
CC(=CCNc1ncnc2c1[nH]cn2)CInChI:
InChI=1S/C10H13N5/c1-7(2)3-4-11-9-8-10(13-5-12-8)15-6-14-9/h3,5-6H,4H2,1-2H3,(H2,11,12,13,14,15)InChIKey:
HYVABZIGRDEKCD-UHFFFAOYSA-NDeepSMILES:
CC=CCNcncncc6[nH]cn5))))))))))))CFunctional groups:
CC=C(C)C, cNC, c[nH]c, cnc
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ncc2[nH]cnc2n1Scaffold Graph/Node level:
C1NCC2NCNC2N1Scaffold Graph level:
C1CCC2CCCC2C1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Imidazopyrimidines
ClassyFire Subclass: Purines and purine derivatives
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Pseudoalkaloids
NP Classifier Class: Purine alkaloids
NP-Likeness score: -0.004
Chemical structure download