Summary
IMPPAT Phytochemical identifier: IMPHY008013
Phytochemical name: Huperzine A
Synonymous chemical names:huperzine a, selagine
External chemical identifiers:CID:854026, ChEMBL:CHEMBL395280, ChEBI:78330, ZINC:ZINC000009411213, FDASRS:0111871I23, SureChEMBL:SCHEMBL16789797, MolPort-035-394-938
Chemical structure information
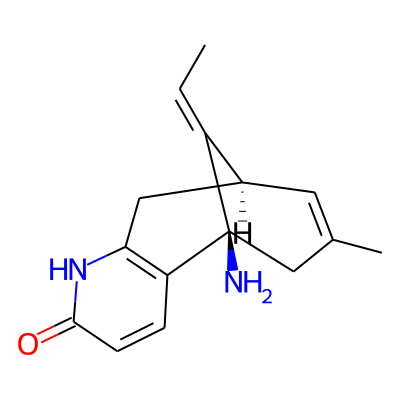
SMILES:
C/C=C/1[C@H]2C=C(C[C@]1(N)c1c(C2)[nH]c(=O)cc1)CInChI:
InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1InChIKey:
ZRJBHWIHUMBLCN-YQEJDHNASA-NDeepSMILES:
C/C=C[C@H]C=CC[C@]6N)ccC8)[nH]c=O)cc6))))))))CFunctional groups:
C/C=C(/C)C, CC(C)=CC, CN, c=O, c[nH]c
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C=C1C2C=CCC1c1ccc(=O)[nH]c1C2Scaffold Graph/Node level:
CC1C2CCCC1C1CCC(O)NC1C2Scaffold Graph level:
CC1CCC2C(C1)CC1CCCC2C1C
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Quinolines and derivatives
ClassyFire Subclass: Quinolones and derivatives
NP Classifier Biosynthetic pathway: Alkaloids, Terpenoids
NP Classifier Superclass: Pseudoalkaloids
NP Classifier Class: Terpenoid alkaloids
NP-Likeness score: 2.321
Chemical structure download