Summary
IMPPAT Phytochemical identifier: IMPHY009559
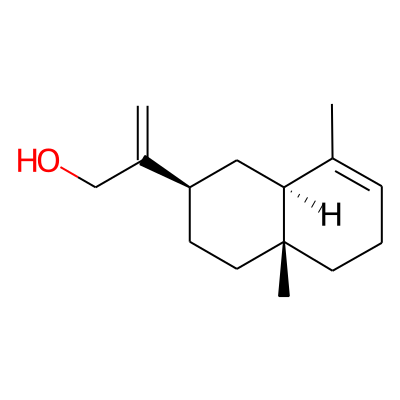
Phytochemical name: 2-Naphthaleneethanol, 1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-beta-methylene-, (2R,4aR,8aR)-
Synonymous chemical names:(-) alpha costol, (-)α-costol
External chemical identifiers:CID:13006421, ZINC:ZINC000138026129
Chemical structure information
SMILES:
OCC(=C)[C@@H]1CC[C@@]2([C@@H](C1)C(=CCC2)C)CInChI:
InChI=1S/C15H24O/c1-11-5-4-7-15(3)8-6-13(9-14(11)15)12(2)10-16/h5,13-14,16H,2,4,6-10H2,1,3H3/t13-,14+,15-/m1/s1InChIKey:
MTJCJJFCDOSALI-QLFBSQMISA-NDeepSMILES:
OCC=C)[C@@H]CC[C@@][C@@H]C6)C=CCC6)))C)))CFunctional groups:
C=C(C)C, CC=C(C)C, CO
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C1=CC2CCCCC2CC1Scaffold Graph/Node level:
C1CCC2CCCCC2C1Scaffold Graph level:
C1CCC2CCCCC2C1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Sesquiterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Sesquiterpenoids
NP Classifier Class: Eudesmane sesquiterpenoids
NP-Likeness score: 3.403
Chemical structure download