Summary
IMPPAT Phytochemical identifier: IMPHY009673
Phytochemical name: Isoboldine
Synonymous chemical names:(+) isoboldine, (+)-isoboldine, (+)isoboldine, isoboldine
External chemical identifiers:CID:133323, ChEMBL:CHEMBL462880, ChEBI:5986, ZINC:ZINC000000898659, FDASRS:M002E511AJ, SureChEMBL:SCHEMBL12140647, MolPort-004-955-365
Chemical structure information
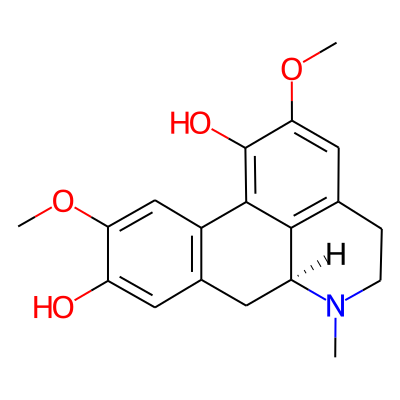
SMILES:
COc1cc2-c3c(O)c(OC)cc4c3[C@H](Cc2cc1O)N(C)CC4InChI:
InChI=1S/C19H21NO4/c1-20-5-4-10-8-16(24-3)19(22)18-12-9-15(23-2)14(21)7-11(12)6-13(20)17(10)18/h7-9,13,21-22H,4-6H2,1-3H3/t13-/m0/s1InChIKey:
LINHZVMHXABQLB-ZDUSSCGKSA-NDeepSMILES:
COccc-ccO)cOC))ccc6[C@H]Cc%10cc%14O)))))NC)CC6Functional groups:
CN(C)C, cO, cOC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccc2c(c1)CC1NCCc3cccc-2c31Scaffold Graph/Node level:
C1CCC2C(C1)CC1NCCC3CCCC2C31Scaffold Graph level:
C1CCC2C(C1)CC1CCCC3CCCC2C31
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Alkaloids and derivativesClassyFire Class: Aporphines
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tyrosine alkaloids
NP Classifier Class: Aporphine alkaloids, Isoquinoline alkaloids
NP-Likeness score: 1.687
Chemical structure download