Summary
IMPPAT Phytochemical identifier: IMPHY010549
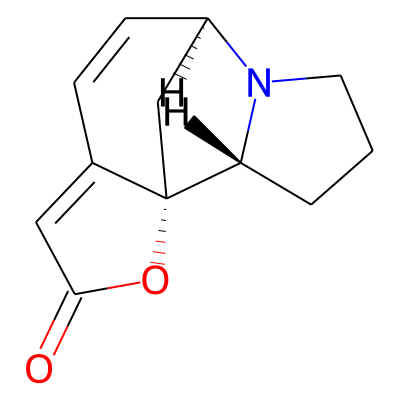
Phytochemical name: Norsecurinine
Synonymous chemical names:nor securinine, nor-securinine, norsecurinine
External chemical identifiers:CID:11106439, ZINC:ZINC000031460486, FDASRS:OPF6A516XH, SureChEMBL:SCHEMBL20558492
Chemical structure information
SMILES:
O=C1C=C2[C@@]3(O1)C[C@@H](C=C2)N1[C@@H]3CCC1InChI:
InChI=1S/C12H13NO2/c14-11-6-8-3-4-9-7-12(8,15-11)10-2-1-5-13(9)10/h3-4,6,9-10H,1-2,5,7H2/t9-,10-,12+/m1/s1InChIKey:
NBGOALXYAZVRPS-FOGDFJRCSA-NDeepSMILES:
O=CC=C[C@@]O5)C[C@@H]C=C6))N[C@@H]5CCC5Functional groups:
CC=CC1=CC(=O)OC1, CN(C)C
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C1C=C2C=CC3CC2(O1)C1CCCN31Scaffold Graph/Node level:
OC1CC2CCC3CC2(O1)C1CCCN31Scaffold Graph level:
CC1CC2CCC3CC2(C1)C1CCCC31
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Pyrrolizidines
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Lysine alkaloids, Tryptophan alkaloids
NP Classifier Class: Quinolizidine alkaloids, Simple indole alkaloids
NP-Likeness score: 3.275
Chemical structure download