Summary
IMPPAT Phytochemical identifier: IMPHY011573
Phytochemical name: Curine
Synonymous chemical names:(+)-curine, 1-bebeerine, 1-curine, curine, l-, hayatine, hayatine (+- curine), l-curine
External chemical identifiers:CID:253793, ChEMBL:CHEMBL1169627, ZINC:ZINC000004098030, FDASRS:6037FRH2SX
Chemical structure information
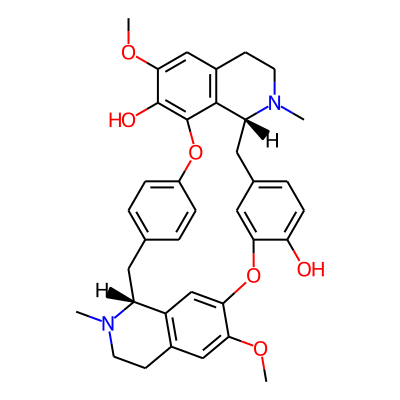
SMILES:
COc1cc2CCN([C@H]3c2cc1Oc1cc(ccc1O)C[C@H]1N(C)CCc2c1c(Oc1ccc(C3)cc1)c(c(c2)OC)O)CInChI:
InChI=1S/C36H38N2O6/c1-37-13-11-23-18-31(41-3)32-20-26(23)27(37)15-21-5-8-25(9-6-21)43-36-34-24(19-33(42-4)35(36)40)12-14-38(2)28(34)16-22-7-10-29(39)30(17-22)44-32/h5-10,17-20,27-28,39-40H,11-16H2,1-4H3/t27-,28-/m1/s1InChIKey:
NGZXDRGWBULKFA-VSGBNLITSA-NDeepSMILES:
COcccCCN[C@H]c6cc%10Occcccc6O))))C[C@H]NC)CCcc6cOccccC%22)cc6)))))))ccc6)OC)))O))))))))))))))))))CFunctional groups:
CN(C)C, cO, cOC, cOc
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1cc2cc(c1)Oc1ccc3c(c1)C(Cc1ccc(cc1)Oc1cccc4c1C(C2)NCC4)NCC3Scaffold Graph/Node level:
C1CC2CC(C1)OC1CCC3CCNC(CC4CCC(CC4)OC4CCCC5CCNC(C2)C54)C3C1Scaffold Graph level:
C1CC2CC(C1)CC1CCCC3CCCC(CC4CCC(CC4)CC4CCCC5CCC(C2)CC54)C31
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organic oxygen compoundsClassyFire Class: Organooxygen compounds
ClassyFire Subclass: Ethers
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tyrosine alkaloids
NP Classifier Class: Isoquinoline alkaloids, Tetrahydroisoquinoline alkaloids
NP-Likeness score: 1.903
Chemical structure download