Summary
IMPPAT Phytochemical identifier: IMPHY011632
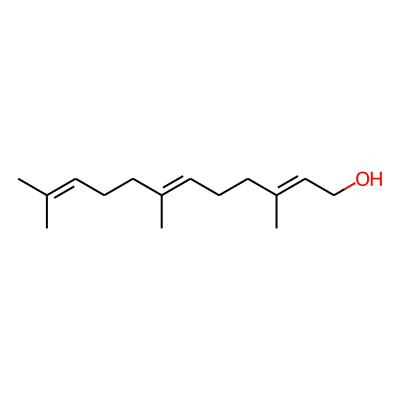
Phytochemical name: Farnesol
Synonymous chemical names:(e)(e)-farnesol, (e)-farnesol, (e, e )-farnesol, (e, e) -farnesol, (e, e)-farnesol, (e, e)-farnesolh, (e,e)-farnesol, e,e-farnesol, farnesol, farnesol
, farnesol*, farnesol+, farnesol, trans-, farnesol,(ee)-, trans farnesol, trans, trans-farnesol, trans,trans-farnesol, trans-farnesol, trans-trans-farnesolExternal chemical identifiers:
CID:445070, ChEMBL:CHEMBL25308, ChEBI:16619, ZINC:ZINC000001532860, FDASRS:X23PI60R17, SureChEMBL:SCHEMBL58068, MolPort-001-769-747
Chemical structure information
SMILES:
OC/C=C(/CC/C=C(/CCC=C(C)C)C)CInChI:
InChI=1S/C15H26O/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-16/h7,9,11,16H,5-6,8,10,12H2,1-4H3/b14-9+,15-11+InChIKey:
CRDAMVZIKSXKFV-YFVJMOTDSA-NDeepSMILES:
OC/C=C/CC/C=C/CCC=CC)C)))))C)))))CFunctional groups:
C/C=C(/C)C, CC=C(C)C, CO
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipidsClassyFire Subclass: SesquiterpenoidsNP Classifier Biosynthetic pathway: TerpenoidsNP Classifier Superclass: Sesquiterpenoids, MonoterpenoidsNP Classifier Class: Acyclic monoterpenoids, Farnesane sesquiterpenoidsNP-Likeness score: 2.191
Chemical structure download