Summary
IMPPAT Phytochemical identifier: IMPHY011633
Phytochemical name: (2Z,6E)-Farnesol
Synonymous chemical names:(2,e)-farnesol, (2z)-(6e)-farnesol, (2z, 6e)-farnesol, (2z,6e) farnesol, (2z,6e)-farnesol, (2z,ge)-farnesol, (z)-(e)-farnesol, (z,e)- farnesol, (z,e)-farnesol, 2(z),6(e)-farnesol, 2z,6e-farnesol, cis trans-farnesol, cis,trans-farnesol, cis-trans farnesol, farnesol,(ze)-, z,e-farnesol
External chemical identifiers:CID:1549108, ChEBI:16774, ZINC:ZINC000013507234, FDASRS:SQ4TI19PXT, SureChEMBL:SCHEMBL806894
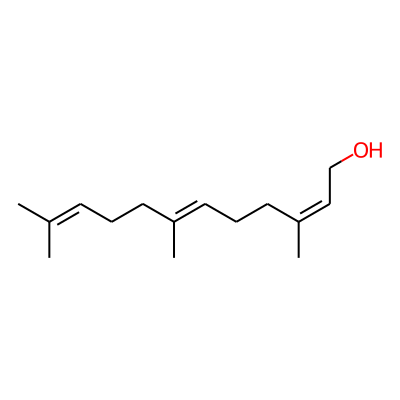
Chemical structure information
SMILES:
OC/C=C(CC/C=C(/CCC=C(C)C)C)/CInChI:
InChI=1S/C15H26O/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-16/h7,9,11,16H,5-6,8,10,12H2,1-4H3/b14-9+,15-11-InChIKey:
CRDAMVZIKSXKFV-PVMFERMNSA-NDeepSMILES:
OC/C=CCC/C=C/CCC=CC)C)))))C)))))/CFunctional groups:
C/C=C(/C)C, C/C=C(C)C, CC=C(C)C, CO
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Sesquiterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Sesquiterpenoids, Monoterpenoids
NP Classifier Class: Acyclic monoterpenoids, Farnesane sesquiterpenoids
NP-Likeness score: 2.191
Chemical structure download