Summary
IMPPAT Phytochemical identifier: IMPHY011691
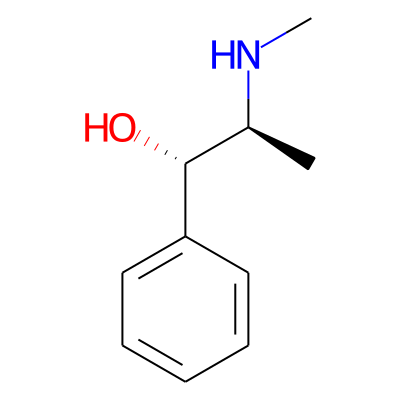
Phytochemical name: Pseudoephedrine
Synonymous chemical names:(+)-isoephedrine, (+)-pseudoephedrine, (+)pseudoephedrine, d-pseudoephedrine, ephedrine, pseudo, ephedrine, pseudo (+), pseudo-ephedrine, pseudoephedrine, pseudoephedrine (+), ψ-ephedrine
External chemical identifiers:CID:7028, ChEMBL:CHEMBL1590, ChEBI:51209, ZINC:ZINC000000020259, FDASRS:7CUC9DDI9F, SureChEMBL:SCHEMBL4368
Chemical structure information
SMILES:
CN[C@H]([C@H](c1ccccc1)O)CInChI:
InChI=1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10+/m0/s1InChIKey:
KWGRBVOPPLSCSI-WCBMZHEXSA-NDeepSMILES:
CN[C@H][C@H]cccccc6))))))O))CFunctional groups:
CNC, CO
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccccc1Scaffold Graph/Node level:
C1CCCCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: BenzenoidsClassyFire Class: Benzene and substituted derivatives
ClassyFire Subclass: Phenylpropanes
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Pseudoalkaloids
NP Classifier Class: Phenylalanine-derived alkaloids
NP-Likeness score: 0.43
Chemical structure download