Summary
IMPPAT Phytochemical identifier: IMPHY011714
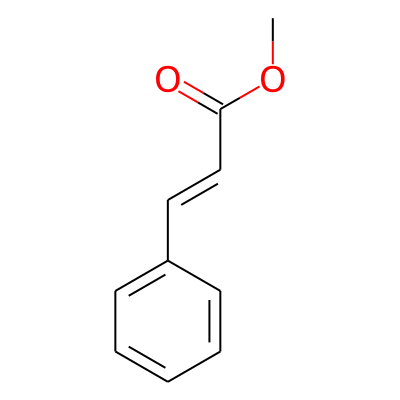
Phytochemical name: Methyl cinnamate
Synonymous chemical names:(e)-methyl cinnamate, (e)-methyl-cinnamate, cinnamic-acid-methyl-ester, me-cinnamate, methyl (e)-cinnamate, methyl (e)-cinnamate (9), methyl cinnamate, methyl(e)-cinnamate, methyl-cinnamate, methylcinnamate, methyltrans-cinnamate, trans-methyl cinnamate
External chemical identifiers:CID:637520, ChEMBL:CHEMBL55060, ChEBI:6857, ZINC:ZINC000000896129, FDASRS:533CV2ZCQL, SureChEMBL:SCHEMBL101530, MolPort-001-783-139
Chemical structure information
SMILES:
COC(=O)/C=C/c1ccccc1InChI:
InChI=1S/C10H10O2/c1-12-10(11)8-7-9-5-3-2-4-6-9/h2-8H,1H3/b8-7+InChIKey:
CCRCUPLGCSFEDV-BQYQJAHWSA-NDeepSMILES:
COC=O)/C=C/cccccc6Functional groups:
c/C=C/C(=O)OC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccccc1Scaffold Graph/Node level:
C1CCCCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Cinnamic acids and derivatives
ClassyFire Subclass: Cinnamic acid esters
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Phenylpropanoids (C6-C3)
NP Classifier Class: Cinnamic acids and derivatives
NP-Likeness score: 0.396
Chemical structure download