Summary
IMPPAT Phytochemical identifier: IMPHY011879
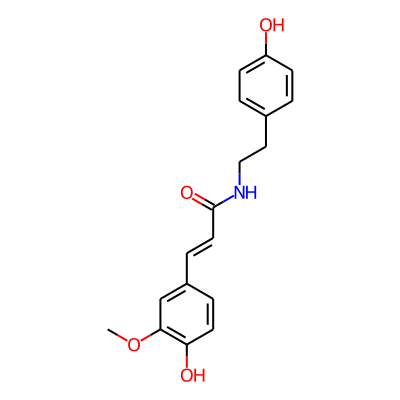
Phytochemical name: Moupinamide
Synonymous chemical names:feruloylthyramine, feruloyltyramine, feruloyltyramine, n-trans, feruloyltyramine, n-trans-, feruloyltyramines, moupinamide, n-feruloyltyramine, n-trans- feruloyl tyramine, n-trans-feruloyl tyramine, n-trans-feruloyltyramine
External chemical identifiers:CID:5280537, ChEMBL:CHEMBL206555, ChEBI:17818, ZINC:ZINC000000901461, SureChEMBL:SCHEMBL1675910, MolPort-001-740-847
Chemical structure information
SMILES:
COc1cc(/C=C/C(=O)NCCc2ccc(cc2)O)ccc1OInChI:
InChI=1S/C18H19NO4/c1-23-17-12-14(4-8-16(17)21)5-9-18(22)19-11-10-13-2-6-15(20)7-3-13/h2-9,12,20-21H,10-11H2,1H3,(H,19,22)/b9-5+InChIKey:
NPNNKDMSXVRADT-WEVVVXLNSA-NDeepSMILES:
COccc/C=C/C=O)NCCcccccc6))O)))))))))))ccc6OFunctional groups:
c/C=C/C(=O)NC, cO, cOC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C(C=Cc1ccccc1)NCCc1ccccc1Scaffold Graph/Node level:
OC(CCC1CCCCC1)NCCC1CCCCC1Scaffold Graph level:
CC(CCCC1CCCCC1)CCC1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: BenzenoidsClassyFire Class: Phenols
ClassyFire Subclass: Methoxyphenols
NP Classifier Biosynthetic pathway: Amino acids and Peptides, Shikimates and Phenylpropanoids
NP Classifier Superclass: Phenylpropanoids (C6-C3)
NP Classifier Class: Cinnamic acid amides
NP-Likeness score: 0.324
Chemical structure download