Summary
IMPPAT Phytochemical identifier: IMPHY013357
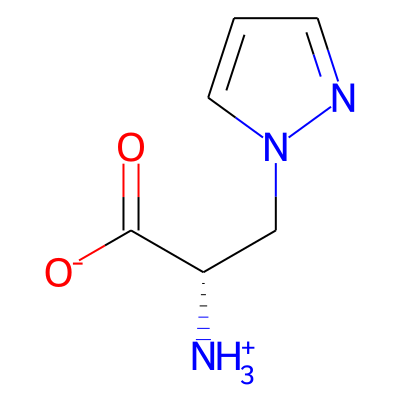
Phytochemical name: (2S)-2-ammonio-3-(1H-pyrazol-1-yl)propanoate
Synonymous chemical names:β-(pyrazol-1-yl)-l-alanine
External chemical identifiers:CID:25203312, ChEBI:57747, ZINC:ZINC000000895518, SureChEMBL:SCHEMBL503649, MolPort-002-499-440
Chemical structure information
SMILES:
[O-]C(=O)[C@H](Cn1cccn1)[NH3+]InChI:
InChI=1S/C6H9N3O2/c7-5(6(10)11)4-9-3-1-2-8-9/h1-3,5H,4,7H2,(H,10,11)/t5-/m0/s1InChIKey:
PIGOPELHGLPKLL-YFKPBYRVSA-NDeepSMILES:
[O-]C=O)[C@H]Cncccn5))))))[NH3+]Functional groups:
CC(=O)[O-], C[NH3+], cnn(c)C
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1cn[nH]c1Scaffold Graph/Node level:
C1CNNC1Scaffold Graph level:
C1CCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organic acids and derivativesClassyFire Class: Carboxylic acids and derivatives
ClassyFire Subclass: Amino acids, peptides, and analogues
NP Classifier Biosynthetic pathway: Amino acids and Peptides
NP Classifier Superclass: Small peptides
NP Classifier Class: Aminoacids
NP-Likeness score: -2.229
Chemical structure download