Summary
IMPPAT Phytochemical identifier: IMPHY013875
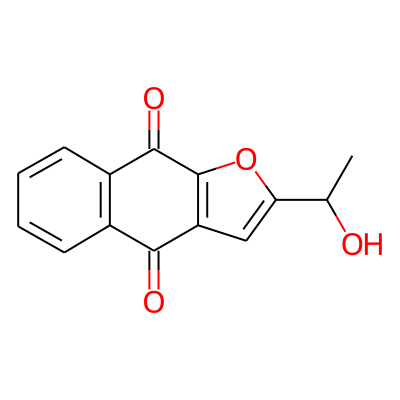
Phytochemical name: 2-(1-Hydroxyethyl)naphtho(2,3-b)furan-4,9-dione
Synonymous chemical names:2-(1-hydroxyethyl)-naphtho[2,3-b] furan-4,9-dione, 2-(1-hydroxyethyl)naphtho[2,3-b]-furan-4,9-dione, 2-(1-hydroxyethyl)naphthol[2,3-b]furan-4,9-dione, 2-(1-hydroxyethylnaphtho[2,3-b]furan-4,9-dione
External chemical identifiers:CID:150068, ChEMBL:CHEMBL294323, SureChEMBL:SCHEMBL1887185
Chemical structure information
SMILES:
CC(c1cc2c(o1)C(=O)c1c(C2=O)cccc1)OInChI:
InChI=1S/C14H10O4/c1-7(15)11-6-10-12(16)8-4-2-3-5-9(8)13(17)14(10)18-11/h2-7,15H,1H3InChIKey:
SAXKEWRSGLPYPB-UHFFFAOYSA-NDeepSMILES:
CCcccco5)C=O)ccC6=O))cccc6)))))))))))OFunctional groups:
CO, cC(c)=O, coc
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C1c2ccccc2C(=O)c2occc21Scaffold Graph/Node level:
OC1C2CCCCC2C(O)C2OCCC12Scaffold Graph level:
CC1C2CCCCC2C(C)C2CCCC12
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Naphthofurans
NP Classifier Biosynthetic pathway: Polyketides
NP Classifier Superclass: Naphthalenes
NP Classifier Class: Naphthoquinones
NP-Likeness score: 1.25
Chemical structure download