Summary
IMPPAT Phytochemical identifier: IMPHY013933
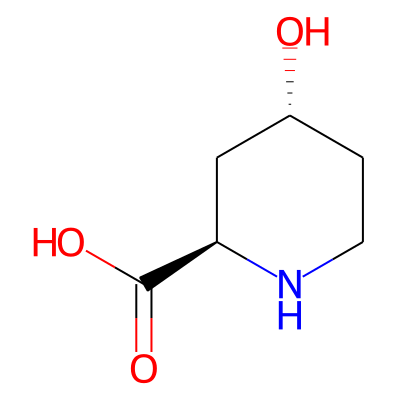
Phytochemical name: (2R,4R)-4-hydroxypiperidine-2-carboxylic acid
Synonymous chemical names:trans-4-hydroxypipecolic acid
External chemical identifiers:CID:261165, ChEMBL:CHEMBL507830, ZINC:ZINC000004933658, SureChEMBL:SCHEMBL1512968
Chemical structure information
SMILES:
O[C@@H]1CCN[C@H](C1)C(=O)OInChI:
InChI=1S/C6H11NO3/c8-4-1-2-7-5(3-4)6(9)10/h4-5,7-8H,1-3H2,(H,9,10)/t4-,5-/m1/s1InChIKey:
KRHNXNZBLHHEIU-RFZPGFLSSA-NDeepSMILES:
O[C@@H]CCN[C@H]C6)C=O)OFunctional groups:
CC(=O)O, CNC, CO
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C1CCNCC1Scaffold Graph/Node level:
C1CCNCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organic acids and derivativesClassyFire Class: Carboxylic acids and derivatives
ClassyFire Subclass: Amino acids, peptides, and analogues
NP Classifier Biosynthetic pathway: Amino acids and Peptides
NP Classifier Superclass: Small peptides
NP Classifier Class: Aminoacids
NP-Likeness score: 1.739
Chemical structure download