Summary
IMPPAT Phytochemical identifier: IMPHY014332
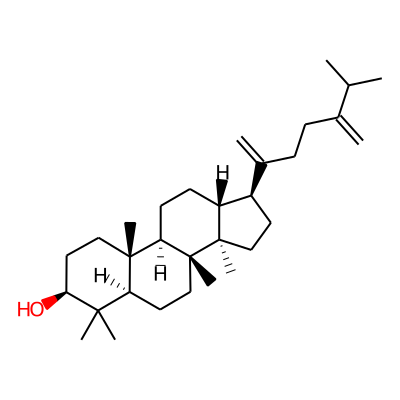
Phytochemical name: 24-Methylenedammarenol
Synonymous chemical names:24-methylenedammarenol
External chemical identifiers:CID:15250873, ZINC:ZINC000096014959
Chemical structure information
SMILES:
C=C(C(C)C)CCC(=C)[C@H]1CC[C@@]2([C@@H]1CC[C@H]1[C@@]2(C)CC[C@@H]2[C@]1(C)CC[C@@H](C2(C)C)O)CInChI:
InChI=1S/C31H52O/c1-20(2)21(3)10-11-22(4)23-14-18-30(8)24(23)12-13-26-29(7)17-16-27(32)28(5,6)25(29)15-19-31(26,30)9/h20,23-27,32H,3-4,10-19H2,1-2,5-9H3/t23-,24-,25+,26-,27+,29+,30-,31-/m1/s1InChIKey:
RBDGMBLJLFFILO-HVCSHQRCSA-NDeepSMILES:
C=CCC)C))CCC=C)[C@H]CC[C@@][C@@H]5CC[C@H][C@@]6C)CC[C@@H][C@]6C)CC[C@@H]C6C)C))O)))))))))))))CFunctional groups:
C=C(C)C, CO
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C1CCC2C(C1)CCC1C3CCCC3CCC21Scaffold Graph/Node level:
C1CCC2C(C1)CCC1C3CCCC3CCC21Scaffold Graph level:
C1CCC2C(C1)CCC1C3CCCC3CCC21
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Lipids and lipid-like moleculesClassyFire Class: Prenol lipids
ClassyFire Subclass: Triterpenoids
NP Classifier Biosynthetic pathway: Terpenoids
NP Classifier Superclass: Triterpenoids
NP Classifier Class: Dammarane and Protostane triterpenoids
NP-Likeness score: 2.916
Chemical structure download