Summary
IMPPAT Phytochemical identifier: IMPHY014908
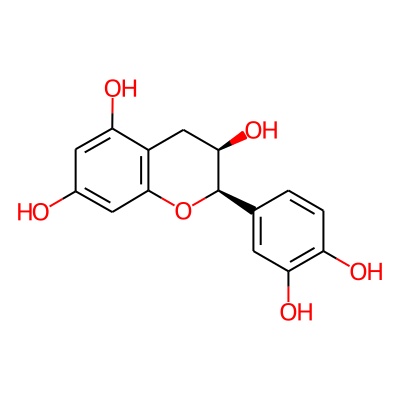
Phytochemical name: (-)-Epicatechin
Synonymous chemical names:(-) epicatechin, (-)-epicatechin, (-)-epicatechol, (-)epicatechin, catechin, epi (-), catechin, epi, (-), catechin,epi (-), catechin,epi(-), catechin,epi,(-), catechol,epi,(-), epi-catechin (-), epicatechin, epicatechin,(-)-, l-epicatechin
External chemical identifiers:CID:72276, ChEMBL:CHEMBL583912, ChEBI:90, ZINC:ZINC000000119988, FDASRS:34PHS7TU43, SureChEMBL:SCHEMBL19412, MolPort-001-740-232
Chemical structure information
SMILES:
Oc1cc2O[C@H](c3ccc(c(c3)O)O)[C@@H](Cc2c(c1)O)OInChI:
InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15-/m1/s1InChIKey:
PFTAWBLQPZVEMU-UKRRQHHQSA-NDeepSMILES:
OcccO[C@H]cccccc6)O))O)))))[C@@H]Cc6cc%10)O))))OFunctional groups:
CO, cO, cOC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccc(C2CCc3ccccc3O2)cc1Scaffold Graph/Node level:
C1CCC(C2CCC3CCCCC3O2)CC1Scaffold Graph level:
C1CCC(C2CCC3CCCCC3C2)CC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Flavonoids
ClassyFire Subclass: Flavans
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Flavonoids
NP Classifier Class: Flavan-3-ols
NP-Likeness score: 2.304
Chemical structure download