Summary
IMPPAT Phytochemical identifier: IMPHY015011
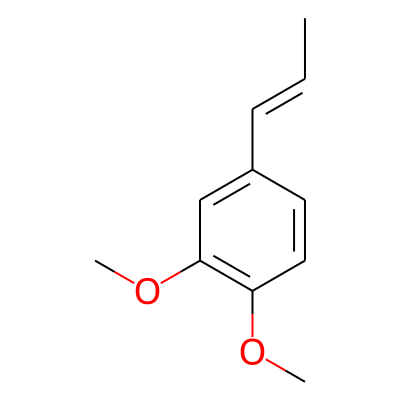
Phytochemical name: Methylisoeugenol
Synonymous chemical names:eugenol iso, methyl ether, methyl isoeugenol, methyl isoeugenol', methyl-isoeugenol, methylisoeugenol, trans-methyl isoeugenol, trans-methylisoeugenol, trans‐methyl isoeugenol
External chemical identifiers:CID:637776, ChEMBL:CHEMBL465829, ChEBI:6877, ZINC:ZINC000000404370, FDASRS:J6M6C71VVR, SureChEMBL:SCHEMBL112937, MolPort-000-698-027
Chemical structure information
SMILES:
C/C=C/c1ccc(c(c1)OC)OCInChI:
InChI=1S/C11H14O2/c1-4-5-9-6-7-10(12-2)11(8-9)13-3/h4-8H,1-3H3/b5-4+InChIKey:
NNWHUJCUHAELCL-SNAWJCMRSA-NDeepSMILES:
C/C=C/cccccc6)OC)))OCFunctional groups:
c/C=C/C, cOC
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccccc1Scaffold Graph/Node level:
C1CCCCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: BenzenoidsClassyFire Class: Benzene and substituted derivatives
ClassyFire Subclass: Methoxybenzenes
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Phenylpropanoids (C6-C3)
NP Classifier Class: Cinnamic acids and derivatives
NP-Likeness score: 0.439
Chemical structure download