Summary
IMPPAT Phytochemical identifier: IMPHY015060
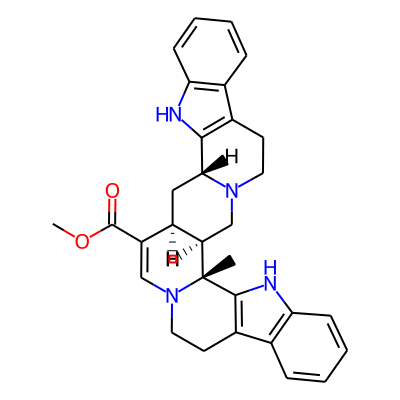
Phytochemical name: Roxburghine B
Synonymous chemical names:roxburghine b
External chemical identifiers:CID:442115, ChEBI:8903, ZINC:ZINC000004097969
Chemical structure information
SMILES:
COC(=O)C1=CN2CCc3c([C@]2([C@H]2[C@@H]1C[C@H]1N(C2)CCc2c1[nH]c1c2cccc1)C)[nH]c1c3cccc1InChI:
InChI=1S/C31H32N4O2/c1-31-24-17-34-13-11-20-18-7-3-5-9-25(18)32-28(20)27(34)15-22(24)23(30(36)37-2)16-35(31)14-12-21-19-8-4-6-10-26(19)33-29(21)31/h3-10,16,22,24,27,32-33H,11-15,17H2,1-2H3/t22-,24-,27-,31-/m1/s1InChIKey:
SHKMVIVFLHPOSB-TVRWTGHXSA-NDeepSMILES:
COC=O)C=CNCCcc[C@]6[C@H][C@@H]%10C[C@H]NC6)CCcc6[nH]cc5cccc6))))))))))))))))C))[nH]cc5cccc6Functional groups:
CN(C)C, COC(=O)C(C)=CN(C)C, c[nH]c
Molecular scaffolds
Scaffold Graph/Node/Bond level:
C1=CN2CCc3c([nH]c4ccccc34)C2C2CN3CCc4c([nH]c5ccccc45)C3CC12Scaffold Graph/Node level:
C1CCC2C(C1)NC1C2CCN2CC3C(CCN4CCC5C6CCCCC6NC5C34)CC12Scaffold Graph level:
C1CCC2C(C1)CC1C3CC4CCC5CCC6C7CCCCC7CC6C5C4CC3CCC21
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Alkaloids and derivativesClassyFire Class: Corynanthean-type alkaloids
NP Classifier Biosynthetic pathway: Alkaloids
NP Classifier Superclass: Tryptophan alkaloids
NP Classifier Class: Corynanthe type
NP-Likeness score: 0.67
Chemical structure download