Summary
IMPPAT Phytochemical identifier: IMPHY015334
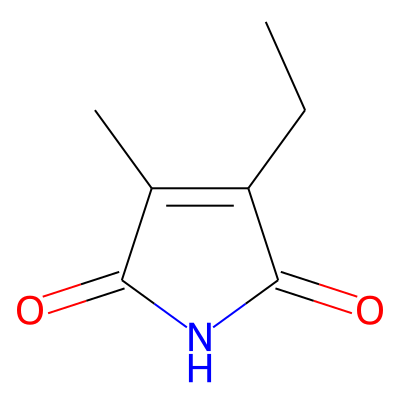
Phytochemical name: 3-Ethyl-4-methyl-1H-pyrrole-2,5-dione
Synonymous chemical names:3-ethyl-4-methyl-1h-pyrrole-2,5- - dione, 3-ethyl-4-methyl-1h-pyrrole-2,5-dione (2-ethyl-3-methylmaleimide)
External chemical identifiers:CID:29995, ZINC:ZINC000005509277, FDASRS:S5G691PF96, SureChEMBL:SCHEMBL6239729
Chemical structure information
SMILES:
CCC1=C(C)C(=O)NC1=OInChI:
InChI=1S/C7H9NO2/c1-3-5-4(2)6(9)8-7(5)10/h3H2,1-2H3,(H,8,9,10)InChIKey:
CUBICSJJYOPOIA-UHFFFAOYSA-NDeepSMILES:
CCC=CC)C=O)NC5=OFunctional groups:
CC1=C(C)C(=O)NC1=O
Molecular scaffolds
Scaffold Graph/Node/Bond level:
O=C1C=CC(=O)N1Scaffold Graph/Node level:
OC1CCC(O)N1Scaffold Graph level:
CC1CCC(C)C1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organoheterocyclic compoundsClassyFire Class: Pyrrolidines
ClassyFire Subclass: Pyrrolidones
NP Classifier Biosynthetic pathway: Alkaloids
NP-Likeness score: 0.535
Chemical structure download