Summary
IMPPAT Phytochemical identifier: IMPHY017075
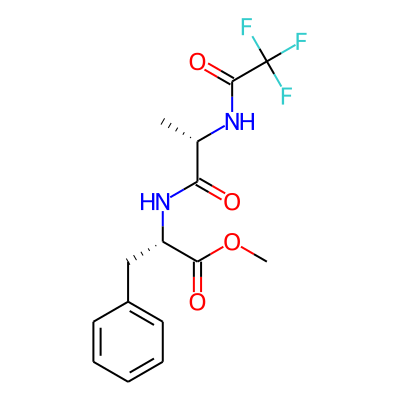
Phytochemical name: N-(Trifluoroacetyl)-L-Ala-L-Phe-OMe
Synonymous chemical names:l-phenylalanine,n-[n-(trifluoroacetyl)-l-alanyl]-,methyl ester
External chemical identifiers:CID:13751946
Chemical structure information
SMILES:
COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)C(F)(F)F)CInChI:
InChI=1S/C15H17F3N2O4/c1-9(19-14(23)15(16,17)18)12(21)20-11(13(22)24-2)8-10-6-4-3-5-7-10/h3-7,9,11H,8H2,1-2H3,(H,19,23)(H,20,21)/t9-,11-/m0/s1InChIKey:
MZLFWWZMQCCXJB-ONGXEEELSA-NDeepSMILES:
COC=O)[C@H]Ccccccc6)))))))NC=O)[C@@H]NC=O)CF)F)F))))CFunctional groups:
CC(=O)NC, CF, CNC(C)=O, COC(C)=O
Molecular scaffolds
Scaffold Graph/Node/Bond level:
c1ccccc1Scaffold Graph/Node level:
C1CCCCC1Scaffold Graph level:
C1CCCCC1
Chemical classification
ClassyFire Kingdom: Organic compoundsClassyFire Superclass: Organic acids and derivativesClassyFire Class: Carboxylic acids and derivatives
ClassyFire Subclass: Amino acids, peptides, and analogues
NP Classifier Biosynthetic pathway: Amino acids and Peptides
NP Classifier Superclass: Small peptides
NP Classifier Class: Dipeptides, Tripeptides
NP-Likeness score: -0.467
Chemical structure download