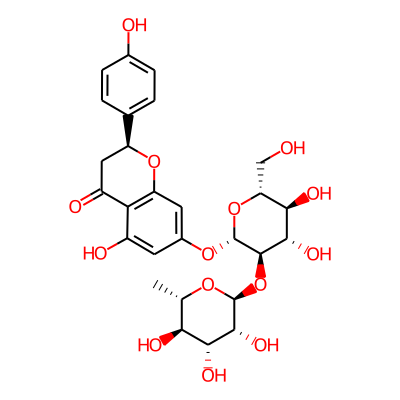
Summary
SMILES: OC[C@H]1O[C@@H](Oc2cc(O)c3c(c2)O[C@@H](CC3=O)c2ccc(cc2)O)[C@@H]([C@H]([C@@H]1O)O)O[C@@H]1O[C@@H](C)[C@@H]([C@H]([C@H]1O)O)OInChI: InChI=1S/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16-,18+,20-,21+,22+,23-,24+,25+,26-,27+/m0/s1InChIKey: DFPMSGMNTNDNHN-ZPHOTFPESA-N
DeepSMILES: OC[C@H]O[C@@H]OcccO)ccc6)O[C@@H]CC6=O)))cccccc6))O)))))))))))))[C@@H][C@H][C@@H]6O))O))O[C@@H]O[C@@H]C)[C@@H][C@H][C@H]6O))O))O
Scaffold Graph/Node/Bond level: O=C1CC(c2ccccc2)Oc2cc(OC3OCCCC3OC3CCCCO3)ccc21
Scaffold Graph/Node level: OC1CC(C2CCCCC2)OC2CC(OC3OCCCC3OC3CCCCO3)CCC12
Scaffold Graph level: CC1CC(C2CCCCC2)CC2CC(CC3CCCCC3CC3CCCCC3)CCC12
Functional groups: CO; CO[C@H](C)OC; cC(C)=O; cO; cOC; cO[C@@H](C)OC
Chemical classification
ClassyFire Kingdom: Organic compounds
ClassyFire Superclass: Phenylpropanoids and polyketidesClassyFire Class: Flavonoids
ClassyFire Subclass: Flavonoid glycosides
NP Classifier Biosynthetic pathway: Shikimates and Phenylpropanoids
NP Classifier Superclass: Flavonoids
NP Classifier Class: Flavanones
Synonymous chemical names:naringin
External chemical identifiers:CID:442428; ChEMBL:CHEMBL451532; ChEBI:28819; ZINC:ZINC000008143604; FDASRS:N7TD9J649B; SureChEMBL:SCHEMBL23432; MolPort-001-742-592
Chemical structure download